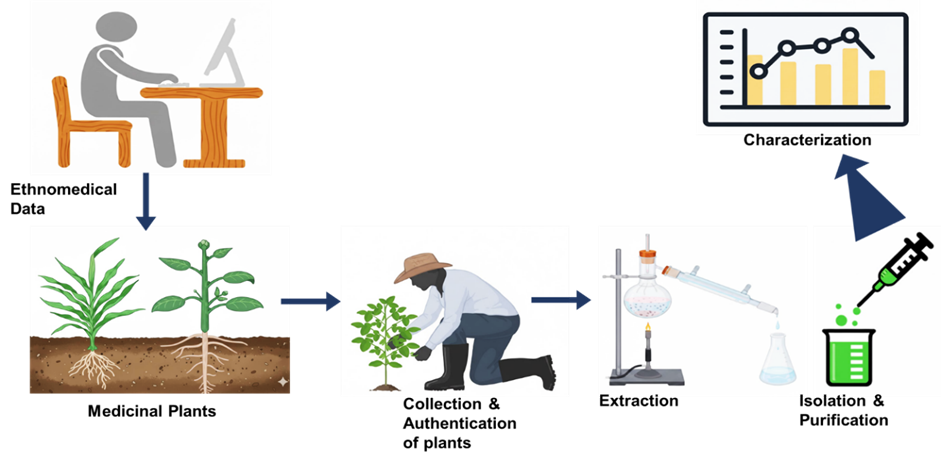
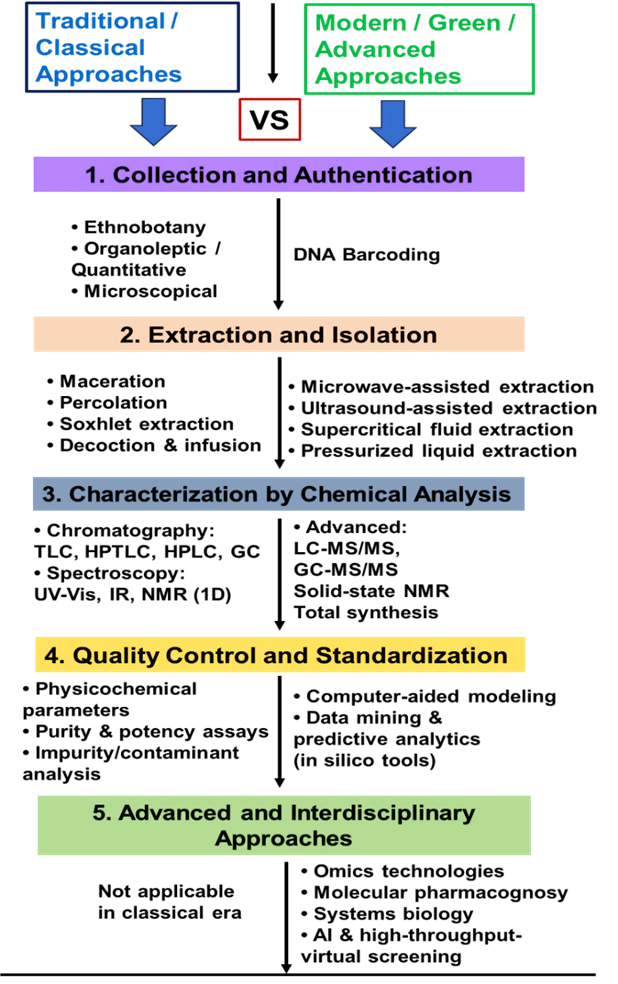
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431-441. doi:10.1016/j.lfs.2005.09.012
- Priya S, Satheeshkumar P. 5 - Natural products from plants: Recent developments in phytochemicals, phytopharmaceuticals, and plant-based neutraceuticals as anticancer agents. Funct Preserv Phytochem. 2020:145-163. https://doi.org/10.1016/B978-0-12-818593-3.00005-1
- Kinghorn AD. Pharmacognosy in the 21st century. J Pharm Pharmacol. 2001;53(2):135-148. doi:10.1211/0022357011775334
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 2011;8(1):1-10. doi:10.4314/ajtcam.v8i1.7
- Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9(11):258. doi:10.3390/metabo9110258
- Lesko LJ, van der Graaf PH. History and evolution of innovations in clinical pharmacology. Clin Pharmacol Ther. Published online September 24, 2025. doi:10.1002/cpt.70048
- Khan RA. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm J. 2018;26(5):739-753. doi:10.1016/j.jsps.2018.01.004
- Thomford NE, Senthebane DA, Rowe A, et al. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int J Mol Sci. 2018;19(6):1578. doi:10.3390/ijms19061578
- Zhen Z, Yin L, Niu T, Rehman A, Liu Y, Zeng K. Target discovery-directed pharmacological mechanism elucidation of bioactive natural products. Med Rev. 2025;5(4):277-296. doi:10.1515/mr-2024-0076
- Yadav S. Transformative frontiers: A comprehensive review of emerging technologies in modern healthcare. Cureus. 2024;16(3):e56538. doi:10.7759/cureus.56538
- World Health Organization. Integrating traditional medicine into health systems. WHO. July 2024. Last retrieved Sept.10, 2025. https://www.who.int/westernpacific/activities/integrating-traditional-medicine-into-national-health-systems
- World Health Organization. Traditional medicine in the WHO South-East Asia Region: review of progress 2014–2019. 29 March 2021. https://www.who.int/publications/i/item/9789290228295
- Abubakar AR, Haque M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci. 2020;12(1):1-10. doi:10.4103/jpbs.JPBS_327_19
- Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Natural products in drug discovery: Advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200-216. doi:10.1038/s41573-020-00114-2
- Wang M, Huang W, Huang J, Luo Y, Huang N. Natural bioactive compounds from herbal medicine in Alzheimer's disease: from the perspective of GSK-3β. Front Pharmacol. 2025;16:1497861. doi:10.3389/fphar.2025.1497861
- Musale P, Patil S, More B, et al. Recent advance of herbal medicines in cancer – a molecular approach. Heliyon. 2023;9(3):e13455. doi:10.1016/j.heliyon.2023.e13455
- Izzo AA, Borrelli F, Capasso R, et al. Natural products and cancer: From drug discovery to prevention and therapy. Br J Pharmacol. 2025;182(5):1107-1125. doi:10.1111/bph.16123
- Chavan RD, Patil RS, Bhosale SR, et al. HIV and the role of various medicinal plants against infection in humans. In: Bioactive Compounds in Cancer Care and Prevention. 2023:15-28.
- Khanyisile M, Thabo M, Sipho D, et al. Herbal formulations exhibit potent in vitro activity against HIV-1 infection. Front Pharmacol. 2025;16:1132. doi:10.3389/fphar.2025.01132
- Vrabec R, Novak P, Jelinek P, et al. Natural alkaloids as multi-target compounds towards factors implicated in Alzheimer’s disease. Int J Mol Sci. 2023;24(9):5123. doi:10.3390/ijms24095123
- She L, Zhao X, Li Y, et al. Ginsenoside RK1 improves cognitive impairments in Alzheimer’s disease. Phytomedicine. 2024;105:154347. doi:10.1016/j.phymed.2024.154347
- ALNasser MN, Alboraiy GM, Alsowig EM, Alqattan FM. Cholinesterase inhibitors from plants and their potential in Alzheimer's treatment: systematic review. Brain Sci. 2025;15(2):215. doi:10.3390/brainsci15020215
- Delves M, Lafuente-Monasterio MJ, Upton L, Ruecker A, Leroy D, Gamo FJ, Sinden R. Fueling open innovation for malaria transmission-blocking drugs: hundreds of molecules targeting early parasite mosquito stages. Front Microbiol. 2019;10:2134. doi:10.3389/fmicb.2019.02134
- Memarzia A, Khazdair MR, Behrouz S, Gholamnezhad Z, Jafarnezhad M, Saadat S, Boskabady MH. Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. Biofactors. 2021 May;47(3):311-350. doi: 10.1002/biof.1716.
- Barrera SD, Cepeda LJB, Báez DAD, Kwon J, Siddiq A, Parra JEC, Marya A, Chaurasia A. Herbal extracts in orofacial pain: a systematic review and direct and indirect meta-analysis. Sci Rep. 2024;14(1):29656. doi:10.1038/s41598-024-77796-7
- Assefa A, Mesfin K, Girmay T. A comprehensive review on animals and their products used in traditional folk medicine in Ethiopia. J Ethnobiol Ethnomed. 2025;21:24. doi:10.1186/s13002-025-00767-3
- Esposito R, Federico S, Bertolino M, Zupo V, Costantini M. Marine Demospongiae: a challenging treasure of bioactive compounds. Mar Drugs. 2022;20(4):244. doi:10.3390/md20040244
- Bolli GB, Cheng AY, Owens DR. Insulin: Evolution of insulin formulations and their application in clinical practice over 100 years. Acta Diabetol. 2022;59(9):1129-1144. doi:10.1007/s00592-022-01906-0
- Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines (Basel). 2018;5(3):93. doi:10.3390/medicines5030093
- Prasathkumar M, Anisha S, Dhrisya C, Becky R, Sadhasivam S. Therapeutic and pharmacological efficacy of selective Indian medicinal plants: a review. Phytomedicine Plus. 2021;1(2):100029. doi:10.1016/j.phyplu.2021.100029
- Shafodino FS, Lusilao JM, Mwapagha LM. Preparation of medicinally active extracts and phytochemical characterization of phytoconstituents from medicinal plants. Nat Prod Res. 2024;38(20):3508-3518. doi:10.1080/14786419.2023.2252976
- Alves RRN, Albuquerque UP. Animals as a source of drugs: bioprospecting and biodiversity conservation. In: Animals in Traditional Folk Medicine: Implications for Conservation. Springer; 2012:67-89.
- Federico S, Bertolino M, Esposito R, et al. Toxigenic effects of sponges and benthic diatoms on marine invertebrates and their possible biotechnological applications. Sci Rep. 2024;14:25325. doi:10.1038/s41598-024-25325-0
- Semlitsch T, Engler J, Siebenhofer A, et al. (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;11:CD005613. doi:10.1002/14651858.CD005613.pub4
- Clardy J, Fischbach MA, Currie CR. The natural history of antibiotics. Curr Biol. 2009;19(11):R437-R441. doi:10.1016/j.cub.2009.04.001
- Aminov R. History of antimicrobial drug discovery: major classes and health impact. Biochem Pharmacol. 2017;133:4-19. doi:10.1016/j.bcp.2017.04.007
- S chaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotoxins. Bacteriol Rev. 1969;33(1):48-71.
- Rinehart KL. Antitumor compounds from tunicates. Med Res Rev. 2000;20(1):1-27. doi:10.1002/(SICI)1098-1128(200001)20:1<1::AID-MED1>3.0.CO;2-A
- Sneader W. Drug discovery: a history. Hoboken, NJ: Wiley; 2005.
- Achan J, Talisuna AO, Erhart A, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi:10.1186/1475-2875-10-144
- Hollman A. Digoxin comes from Digitalis purpurea. BMJ. 1996;312(7039):912. doi:10.1136/bmj.312.7039.912
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17(10):1217-1220. doi:10.1038/nm.2471
- Spencer JP. Flavonoids: modulators of brain function? Br J Nutr. 2008;99(E Suppl 1):ES60-ES77. doi:10.1017/S0007114508965776
- Quianzon CC, Cheikh I. History of insulin. J Community Hosp Intern Med Perspect. 2012;2(2). doi:10.3402/jchimp.v2i2.18701
- McGivern JG. Ziconotide: a review of its pharmacology and use in the treatment of pain. Neuropsychiatr Dis Treat. 2007;3(1):69-85. doi: 10.2147/nedt.2007.3.1.69
- Schwartsmann G, Da Rocha AB, Berlinck RG, Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001;2(4):221-225. doi:10.1016/S1470-2045(00)00292-8
- Fleming A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol. 1929;10(3):226-236.
- Waksman SA. Streptomycin: background, isolation, properties, and utilization. Science. 1947;106(2757):447-452. doi:10.1126/science.106.2757.447
- Borel JF, Feurer C, Gubler HU, Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6(4):468-475. doi:10.1007/BF01973261
- Arcamone F, Cassinelli G, Fantini G, et al. Adriamycin, a new antibiotic with antitumor activity. Biotechnol Bioeng. 1969;11(6):1101-1110. doi:10.1002/bit.260110607
- Pirintsos S, Panagiotopoulos A, Bariotakis M, et al. From traditional ethnopharmacology to modern natural drug discovery: a methodology discussion and specific examples. Molecules. 2022;27(13):4060. doi:10.3390/molecules27134060
- Cahlíková L, Šafratová M, Hošťálková A, et al. Pharmacognosy and its role in the system of profile disciplines in pharmacy. Nat Prod Commun. 2020;15(9). doi:10.1177/1934578X20945450
- Davis CC, Choisy P. Medicinal plants meet modern biodiversity science. Curr Biol. 2024;34(4):R158-R173. doi:10.1016/j.cub.2023.12.038
- Chen S, Yin X, Han J, Sun W, Yao H, Song J, Li X. DNA barcoding in herbal medicine: retrospective and prospective. J Pharm Anal. 2023;13(5):431-441. doi:10.1016/j.jpha.2023.03.008
- Orhan IE. Pharmacognosy: science of natural products in drug discovery. Bioimpacts. 2014;4(3):109-110. doi:10.15171/bi.2014.001
- Bitwell C, Indra SS, Luke C, Maseka KK. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci Afr. 2023;19:e01585. doi:10.1016/j.sciaf.2023.e01585
- Zhu F, Zhao B, Hu B, Zhang Y, Xue B, Wang H, Chen Q. Review of available “extraction + purification” methods of natural ceramides and their feasibility for sewage sludge analysis. Environ Sci Pollut Res Int. 2023;30(26):68022-68053. doi:10.1007/s11356-023-26900-x
- Christian OE, Perry DA, Telchy AI, Walton PN, Williams D. Bioactive compounds isolated from a marine sponge selectively inhibit Neisseria gonorrhoeae. Antibiotics (Basel). 2024;13(12):1229. doi:10.3390/antibiotics13121229
- Devi S. Advancements in quantitative and qualitative methods for quality control of herbal drugs: a comprehensive review. Pharmacogn Res. 2025;17(2):411-415. doi: 10.5530/pres.20252077
- Zou L, Li H, Ding X, Liu Z, He D, Kowah JAH, et al. A review of the application of spectroscopy to flavonoids from medicine and food homology materials. Molecules. 2022;27(22):7766. doi:10.3390/molecules27227766
- Balayssac S, Assemat G, Danoun S, Malet-Martino M, Gilard V. Quantitative 1H and 13C NMR and chemometric assessment of 13C NMR data: application to anabolic steroid formulations. Molecules. 2025;30(9):2060. doi:10.3390/molecules30092060
- Orhan IE. Pharmacognosy: science of natural products in drug discovery. Bioimpacts. 2014;4(3):109-110. doi:10.15171/bi.2014.001
- Vera W, Salvador-Reyes R, Quispe-Santivañez G, Kemper G. Detection of adulterants in powdered foods using near-infrared spectroscopy and chemometrics: recent advances, challenges, and future perspectives. Foods. 2025;14(18):3195. doi:10.3390/foods14183195
- Bārzdiņa A, Paulausks A, Bandere D, Brangule A. The potential use of herbal fingerprints by means of HPLC and TLC for characterization and identification of herbal extracts and the distinction of Latvian native medicinal plants. Molecules. 2022;27(8):2555. doi:10.3390/molecules27082555
- Khan S, Alam S, Siddiqui N, et al. Heavy metals, aflatoxins, pesticide residues and microbial load determination in designed polyherbal formulation used against uterine fibroid. Discov Chem. 2025;2:230. doi:10.1007/s44371-025-00314-9
- Dhami N. Trends in pharmacognosy: a modern science of natural medicines. J Herb Med. 2013;3(4):123-131. doi:10.1016/j.hermed.2013.06.001
- Bian X, Qiu Y, Zhao X, Wei H, Zhang Y, Zhang W, et al. Multi-omics dissection of metabolic and transcriptional regulation underlying fruit maturation in Panax ginseng. BMC Plant Biol. 2025;25:1133. doi:10.1186/s12870-025-07221-2
- Rajaei F, Minoccheri C, Wittrup E, Wilson RC, Athey BD, Omenn GS, et al. AI-based computational methods in early drug discovery and post market drug assessment: a survey. IEEE Trans Comput Biol Bioinform. 2025;22(1):97-115. doi:10.1109/TCBB.2024.3492708
- Pathan I, Raza A, Sahu A, Joshi M, Sahu Y, Patil Y, Raza MA, Ajazuddin. Revolutionizing pharmacology: AI-powered approaches in molecular modeling and ADMET prediction. Med Drug Discov. 2025;28:100223. doi:10.1016/j.medidd.2025.100223
- Patridge E, et al. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21(2):204-207. doi:10.1016/j.drudis.2015.11.001
- WHO. Biodiversity. February 2025. https://www.who.int/news-room/fact-sheets/detail/biodiversity
- Fisch C. William Withering: an account of the foxglove and some of its medical uses 1785-1985. J Am Coll Cardiol. 1985;5(5 Suppl A):1A-2A. doi:10.1016/s0735-1097(85)80456-3
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327.
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17(10):1217-1220. doi:10.1038/nm.2471
- Barsa JA, Kline NS. Reserpine in the treatment of psychotics with convulsive disorders. AMA Arch Neurol Psychiatry. 1955;74(1):31-35. doi:10.1001/archneurpsyc.1955.02330130033005
- McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother Res. 2006;20(8):619-633. doi:10.1002/ptr.1936
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363-398.
- Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi:10.1001/jama.2015.6358
- Linde K, Berner MM, Kriston L. St John's wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448. doi:10.1002/14651858.CD000448.pub2
- El-Saadony MT, Yang T, Korma SA, et al. Impacts of turmeric and its principal bioactive curcumin on human health: pharmaceutical, medicinal, and food applications: a comprehensive review. Front Nutr. 2023;9:1040259. doi:10.3389/fnut.2022.1040259
- Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966;88(16):3888-3890. doi:10.1021/ja00964a072
- Messina M. Insights gained from 20 years of soy research. J Nutr. 2010;140(12):2289S-2295S. doi:10.3945/jn.110.124154
- Heber D. Asian foods and herbs: how to use them in diet therapy. J Nutr. 2004;134(6):1333-1336. doi:10.1093/jn/134.6.1333
- Tyler VE, Brady LR, Robbers JE. Pharmacognosy. Philadelphia, PA: Lea & Febiger; 1988.
- Kress WJ, Erickson DL, Jones FA, et al. Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci U S A. 2005;102(23):8369-8374. doi:10.1073/pnas.0503123102
- Cicero AFG, Fogacci F, Banach M. Red yeast rice for hypercholesterolemia. Methodist Debakey Cardiovasc J. 2019;15(3):192-199. doi:10.14797/mdcj-15-3-192
- Bonam SR, Sekar M, Guntuku GS, Nerella SG, Pawar AK, Challa SR, et al. Role of pharmaceutical sciences in future drug discovery. Future Drug Discov. 2021;3(3). doi:10.4155/fdd-2021-0005
- Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200-216. doi:10.1038/s41573-020-00114-z
- Ziemert N, Alanjary M, Weber T, et al. The evolution of genome mining in microbes – a review. Nat Prod Rep. 2016;33(8):988-1005. doi:10.1039/c6np00009c
- Heinrich M, Barnes J, Gibbons S, et al. Best practice in research: overcoming common challenges in phytopharmacological research. J Ethnopharmacol. 2020;246:112230. doi:10.1016/j.jep.2019.112230
- Cravens A, Payne J, Smanski MJ, et al. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat Commun. 2019;10:2142. doi:10.1038/s41467-019-10103-5
- Blin K, Shaw S, Kloosterman AM, et al. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021;49(W1):W29-W35. doi:10.1093/nar/gkab335
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 1981 to 2019. J Nat Prod. 2020;83(3):770-803. doi:10.1021/acs.jnatprod.9b01285
- Medema MH, Fischbach MA. Computational approaches to natural product discovery. Nat Chem Biol. 2015;11(9):639-648. doi:10.1038/nchembio.1884
- Nazar N, Saxena A, Sebastian A, Slater A, Sundaresan V, Sgamma T. Integrating DNA barcoding within an orthogonal approach for herbal product authentication: a narrative review. Phytochem Anal. 2025;36:7-29. doi:10.1002/pca.3466
- Wolfender JL, Marti G, Thomas A, et al. Metabolomics in pharmacognosy: towards the discovery of new bioactive compounds. Curr Opin Chem Biol. 2019;51:104-112. doi:10.1016/j.cbpa.2019.03.001
- Leonti M, Casu L. Traditional medicines and globalization: the future of ancient systems of medicine. Evid Based Complement Alternat Med. 2013;2013:803650. doi:10.1155/2013/803650
- Cordell GA. Ecopharmacognosy: exploring the chemical and biological potential of nature for sustainable drug discovery. Planta Med. 2015;81(16):1455-1463. doi:10.1055/s-0035-1558212
- Zeng X, Zhang P, He W, et al. NPASS: natural product activity and species source database for natural product research. Nucleic Acids Res. 2020;48(D1):D1207-D1212. doi:10.1093/nar/gkz951
- Zhang R, Zhu X, Bai H, et al. Network pharmacology: a new approach for Chinese herbal medicine research. Evid Based Complement Alternat Med. 2021;2021:6681032. doi:10.1155/2021/6681032
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682-690. doi:10.1038/nchembio.118
- Shen B. A new golden age of natural products drug discovery. Cell. 2015;163(6):1297-1300. doi: 10.1016/j.cell.2015.11.031
- Georgiev MI, Weber J, Maciuk A, et al. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol. 2018;102(17):7315-7334. doi:10.1007/s00253-018-9161-2
- Smanski MJ, Zhou H, Claesen J, et al. Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Microbiol. 2016;14(3):135-149. doi:10.1038/nrmicro.2015.24
- Ober D. Nagoya Protocol and its implications on natural product research. Phytochem Rev. 2019;18(3):729-739. doi:10.1007/s11101-019-09604-0
- Chemat F, Abert-Vian M, Fabiano-Tixier AS, et al. Green extraction of natural products: concept and principles. Int J Mol Sci. 2020;21(14):5158. doi:10.3390/ijms21145158
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27(1):1-93. doi: 10.1016/j.mam.2005.07.008
- Leonti M, Casu L. Traditional medicines and globalization: current and future perspectives in ethnopharmacology. Front Pharmacol. 2013; 4:92. doi:10.3389/fphar.2013.00092
- Elufioye TO, Badal S. Background to pharmacognosy. In: Badal S, Delgoda R, eds. Pharmacognosy. Boston, MA: Academic Press; 2017:3–13.
- Sarker SD. Pharmacognosy in modern pharmacy curricula. Pharmacogn Mag. 2012;8(30):91-92. doi:10.4103/0973-1296.93305
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014; 4:177. doi:10.3389/fphar.2013.00177
- Bareetseng S. The worldwide herbal market: trends and opportunities. J Biomed Res Environ Sci. 2022;3(5):575-584. doi:10.37871/jbres1482
- Ahmed F, Khan R, Fatima S, et al. Strengthening the bridge between academic and the industry through the academia-industry collaboration plan design model. Front Psychol. 2022; 13:875940. doi:10.3389/fpsyg.2022.875940
- Majumder S, Panigrahi GK. Advancements in contemporary pharmacological innovation: mechanistic insights and emerging trends in drug discovery and development. Intell Pharm. 2025;3(2):118-126.
- An Q, Li J, Wang H, et al. New strategies to enhance the efficiency and precision of drug discovery. Front Pharmacol. 2025; 16:1550158. doi:10.3389/fphar.2025.1550158
- Luo Z, Chen X, Zhang H, et al. Progress in approved drugs from natural product resources. Chin J Nat Med. 2024;22(3):195-211. doi:10.1016/S1875-5364(23)60252-0
- Ongaro E, Di Mascio F, Natalini A. How the European Union responded to populism and its implications for public sector reforms. Glob Public Policy Gov. 2022;2(1):89-109. doi:10.1007/s43508-022-00036-x
- Shinde V, Dhalwal K. Pharmacognosy: the changing scenario. Pharmacogn Rev. 2007;1(1):1-6. doi:10.4103/0973-7847.56252
- Kumar S, Sharma P, Singh R, et al. Developing a competency assessment framework for pharmacists in primary health care settings in India. PLoS One. 2025;20(3):e0316646. doi: 10.1371/journal.pone.0316646
- Orhan IE. Pharmacognosy: science of natural products in drug discovery. Bioimpacts. 2014;4(3):109-110. doi:10.15171/bi.2014.001
- Roy MK, Roy S, Binduraz B, Afrin L, Haque MA. Pesticide-associated Health and Environmental Risks and the Potential of Biofertilizers in Sustainable Agriculture. J Biosci Public Health. 2025;1(3):16-27. doi.org/10.5455/JBPH.2025.12
- Gogoi D, Borkotoky S, Sharma R, et al. A systemic investigation of the phytochemical space in polyherbal formulations of India: a curated database. Comput Biol Med. 2025;196:110760. doi: 10.1016/j.compbiomed.2025.110760
- Balogun FO, Adeyemi AO, Oladipo OO, et al. Pharmacognosy: importance and applications. In: Pharmacognosy: Medicinal Plants. 2019:289.
- Rahmani AM, Alavi SS, Amini M, et al. Artificial intelligence approaches and mechanisms for big data analytics: a systematic study. PeerJ Comput Sci. 2021;7: e488. doi:10.7717/peerj-cs.488
- Raj A, Jhariya MK. Conservation and sustainable uses of medicinal plants phytochemicals. In: Herbal Medicine Phytochemistry: Applications and Trends. Singapore: Springer; 2024:1825–1852.
- Q, Li J, Wang Y, et al. Advances in analytical techniques for bioactive compound quantification in medicinal plants: innovations, challenges, and pharmaceutical applications. Microchem J. 2025; 214:114119. doi: 10.1016/j.microc.2025.114119
- Efferth T, Banerjee M, Paul NW, et al. Biopiracy of natural products and good bioprospecting practice. Phytomedicine. 2016;23(2):166-173. doi:10.1016/j.phymed.2015.12.006
- Kamau EC, Fedder B, Winter G. The Nagoya Protocol on access to genetic resources and benefit sharing: what is new and what are the implications for provider and user countries and the scientific community. Law Environ Dev J. 2010; 6:246.
- Nanda S. Integrating traditional and contemporary systems for health and well-being. Ann Neurosci. 2023;30(2):77–78. doi:10.1159/000531394
- Karthikeyan A, Joseph A, Nair BG. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol. 2022;20(1):14. doi:10.1186/s43141-022-00317-5
- Gagare S, Patil P, Jain A. Natural product-inspired strategies towards the discovery of novel bioactive molecules. Future J Pharm Sci. 2024;10(1):55. doi:10.1186/s43094-024-00315-7
- Nasim N, Sandeep IS, Mohanty S. Plant-derived natural products for drug discovery: current approaches and prospects. Nucleus (Calcutta). 2022;65(3):399–411.
- Gross EM. Aquatic chemical ecology meets ecotoxicology. Aquat Ecol. 2022;56(2):493–511. doi:10.1007/s10452-022-09994-8
- Ezike TC, Okoye OS, Chukwuma CE, et al. Advances in drug delivery systems, challenges and future directions. Heliyon. 2023;9(6): e17488. doi: 10.1016/j.heliyon.2023.e17488
- Trucillo P. Biomaterials for drug delivery and human applications. Materials (Basel). 2024;17(2):456. doi:10.3390/ma17020456
- Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Am J Trop Med Hyg. 2002;67(1):5–9. doi:10.4269/ajtmh.2002.67.5
 access
access