J. Biosci. Public Health. 2025; 1(3)
Obesity and metabolic complications are closely linked to non-alcoholic fatty liver disease (NAFLD), and the waist-to-hip ratio (WHR) is a simple and accurate measure of overall obesity and risk of metabolic disease. This cross-sectional study attempted to determine gender-based differences within a Bangladeshi population and assess the association between WHR and metabolic complications among NAFLD patients. 200 NAFLD patients in all were enrolled, with 71% being female and 29% being male. Anthropometric and biochemical parameters including body mass index (BMI), fasting blood sugar (FBS), triglyceride levels, and WHR were analyzed. The prevalence of obesity was 89.5%, with males having a higher WHR (0.89±0.026) and females having a higher mean BMI (32.92±2.55 kg/m²). Females experienced metabolic complications at a higher rate (65%) than males (11%). Triglyceride level (p=0.028) was significantly linked to the severity of NAFLD, whereas WHR (p=0.001), BMI (p=0.015), and FBS (p=0.007) were significant predictors of metabolic complications based on logistic regression. According to the results, the main predictors of metabolic disorders in NAFLD patients are increased WHR, BMI, and impaired glucose levels. Early screening and management of central obesity and glycemic control may help reduce disease burden and prevent progression of metabolic complications.
Non-alcoholic fatty liver disease (NAFLD) is a type of liver condition that varies from simple fat accumulation to steatohepatitis, characterized by varying levels of inflammation and fibrosis, and can advance to severe liver disease, including cirrhosis and liver cancer [1]. NAFLD is viewed as the primary contributor to liver-related health issues and death [2]. In contrast to alcoholic liver disease, NAFLD has become increasingly common due to the swift increase in obesity rates, and abnormal liver function tests may occur as a result of NAFLD [3, 4]. Typically, the development of NAFLD has been associated initially with factors that result in hepatic steatosis, followed by a "second hit" that facilitates the progression of liver injury. The production of hepatic triglycerides requires dietary free fatty acids (FFA) that are released from fat stores. In hepatocytes, these fatty acids either undergo oxidation or are converted into triglycerides [5]. The accumulation of triglycerides in the liver and other tissues is caused by a disruption in the processes of fatty acid uptake, synthesis, export, and oxidation [6]. The occurrence of NAFLD ranges from 15% to 30% in the general population, while it affects nearly 50% to 90% of individuals classified as obese. This rate of occurrence is linked to the levels of obesity. Specifically, hepatic steatosis is observed in 65% of individuals with grade I and grade II obesity (BMI = 30-39.9 kg/m²) and in 85% of those who have grade III obesity (BMI = 40-59 kg/m²) [7].
Metabolism refers to the chemical processes in the body that convert nutrients from food into energy. When these normal metabolic pathways are disrupted by disease, medication, therapy, or other external factors, it can lead to various problems known as metabolic complications. These complications commonly involve imbalances or disturbances in the levels of electrolytes, hormones, nutrients, and other substances that are needed for various bodily functions. These imbalances can lead to metabolic complications, like hyperglycemia, liver dysfunction, and electrolyte imbalances [8]. WHO diagnostic criteria of metabolic complications are abdominal obesity (waist-to-hip ratio > 0.9 in men or > 0.85 in women, or body mass index (BMI) > 30 kg/m²). Triglyceride of 150 mg/dl or greater and/or high-density lipoprotein (HDL) cholesterol < 40 mg/dl in men and < 50 mg/dl in women [9].
Metabolic syndrome (MetS) a distinct cluster of metabolic abnormalities, often serves as both a precursor and a major contributor to many metabolic complications, including insulin resistance, type 2 diabetes, cardiovascular disease, and nonalcoholic fatty liver disease. Therefore, recognizing this interrelationship is important [10, 11]. In 2005, the American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI) made slight revisions to the ATP III criteria. As a result, metabolic syndrome is diagnosed when three or more of the following components are present: abdominal obesity (waist circumference exceeding 102 cm in men and 88 cm in women), high triglycerides levels (greater than 150 mg/dl or receiving treatment for high triglycerides), low HDL-C levels (less than 40 mg/dl in men and less than 50 mg/dl in women or undergoing treatment for low HDL-C), hypertension (systolic blood pressure over 130 mmHg or diastolic blood pressure over 85 mmHg or on antihypertensive medication), and impaired fasting glucose (ranging from 100 to 125 mg/dl or receiving treatment for diabetes) [12]. The worldwide prevalence of MetS ranges from 12.5% to 31.4%. is an urgent public health concern that necessitates investigation into possible risk factors [13]. A systematic review and meta-analysis found that the prevalence of metabolic syndrome in the Bangladeshi population is 37.0% [14]. NAFLD may be viewed as the liver manifestation of metabolic syndrome [15].
The onset of NAFLD is closely linked to metabolic syndrome, as evidenced by the observation that nearly 90% of individuals with NAFLD exhibit multiple features of metabolic syndrome, and around 33% meet three or more criteria [16].
In this consideration, the WHR has gained attention as a simple yet effective anthropometric marker of central obesity and metabolic risk. This cross-sectional study was conducted to assess the correlation between WHR and metabolic complications among patients with NAFLD and to explore gender-based differences in this relationship within the Bangladeshi population. Early identification and management of these conditions may help prevent severe outcomes such as hepatic fibrosis and hepatocellular carcinoma. Moreover, it may help to reduce patient disability and economic burden on affected individuals and minimize the overall stress on healthcare systems.
2.1. Study Design and Population
The sample was selected purposively to represent the general population of Bangladesh irrespective of urban and rural areas. A total number of 200 NAFLD participants were recruited in this study. Inclusion criteria of the study were participants having non-alcoholic fatty liver disease. The exclusion criteria are patients with a history of jaundice or who are HBsAg positive and hepatitis-C positive and patients with a history of the following drug intakes: steroids, synthetic estrogens, heparin, calcium channel blockers, amiodarone, valproic acid, arsenic, mercury, homeopathic drugs, ayurvedic drugs, and antiviral agents, and pregnant women.
2.2. Sample Size Calculation
Sample size is calculated by following formula: n=Z2pq/d2 [17].
n = (1.96)2 *0.2*(1-0.2)/ (0.05)2
n = 246
Here: Z =1.96 (value of standard variate at a given confidence interval of 95%)
p = Assumed proportion of NAFLD in metabolic syndrome participants in Bangladesh =20%
q = (1-p)
d = Standard Error 0.05
Considering nonresponse and unavailability of 10% of total participants, it is considered 200.
2.3. Data Collection
The data of the study participants were collected from the outpatient department of Bangladesh Institute of Health Sciences General Hospital (BIHS), Mirpur, Dhaka, Bangladesh. Informed written consent was obtained from each individual participant, and data was collected using a questionnaire. The questionnaire includes socio-demographic information such as age, sex, marital status, residential area, educational status, any history of past illness or any chronic diseases, family history, history of addiction, present or past medication that may elicit liver disease, anthropometric measurement, and biochemical parameters such as serum triglyceride (TG) level and fasting blood sugar level.
2.4. Physical Examination
The body mass index (BMI) of the participants was calculated in kg/m² using a standard formula. BMI = Weight (in kg)/Height (in m²). Waist circumference was measured to the nearest 0.5 cm with a soft non-elastic measuring tape. The waist circumference was taken to the nearest standing horizontal circumference between the lower border of the 12th rib and the highest point of the iliac crest on the mid-axillary line at the end of the normal expiration. Hip circumference was measured at the maximum circumference over the buttocks using soft non-elastic measuring tape, and the reading was taken to the nearest 0.5 cm. Waist-to-hip ratio (WHR) of the study participants was calculated as the ratio of the waist circumference divided by hip circumference.
2.5. Detection of Fatty Liver
Ultrasonography (USG) of the hepatobiliary system was employed to identify the existence or nonexistence of NAFLD. USG is regarded as a readily accessible, economical, and fundamentally noninvasive approach for the identification of NAFLD [18-20]. The doctors utilized a sonographic device with 3.5 MHz transducers to scan the liver, biliary system, spleen, and kidneys. Fatty liver was identified through ultrasound observations indicating that the liver's echogenicity exceeds that of the renal cortex; intrahepatic vessels were not clearly visible; the ultrasound beam showed increased attenuation at the back; and visualization of the diaphragm was inadequate. Since a cirrhotic liver can also exhibit high echogenicity, it was ruled out through medical history, physical assessments, and ultrasound findings such as the coarse echo texture of the liver [21, 22].
2.6. Detection of Metabolic Complications
Metabolic complications were detected by WHO criteria: the World Health Organization (WHO) recommends a WHR of at least 0.90 for men and 0.85 for women to indicate a significantly increased risk of metabolic complications. A WHR greater than 1.0 for either sex indicates an even higher risk [23].
2.7. Analytical Methods
After overnight fasting (8-12 hours), ~6 ml of venous blood was collected between 8.00 and 9.00 am by venipuncture following standard procedure. The blood sample was maintained at 40°C until separation, and the serum was kept at -80°C within an hour of sample collection. Serum was not allowed to be thawed until the assay was performed. Tests for serum glucose and serum triglyceride were performed using the photometric colorimetric method.
2.8. Statistical Analysis
Statistical analyses were performed using SPSS version 27 on Windows 11. Data were expressed as mean ± SD. Associations between categorical variables were assessed using the exact chi-square (χ²) test. Binary logistic regression analysis and ordinal logistic regression were performed to analyze the prediction of metabolic risk. A p-value < 0.05 was considered statistically significant.
Table 1 shows the frequency and distribution of gender, sociodemographic, and clinical characteristics among 200 NAFLD patients. Most of the participants were women (71%), whereas male participants were 29%. The age of NAFLD patients is divided into five age groups. Those aged between 18 and 28 years made up 7.5% (n=15), while those aged 29 to 38 years accounted for 15.0% (n=30). The largest proportion of participants are in the 39 to 48 years’ age group, comprising 30.5% (n=61), and nearly half of the participants (47.0%, n=94) are over 48 years of age.
Table 1. Frequency and distribution of gender, sociodemographic, and clinical characteristics.
Sociodemographic and health related parameters | Variables | Frequency | Percentage (%) |
| Gender | Male | 58 | 29 |
| Female | 142 | 71 | |
| Age (years) | Adult (18-28) | 15 | 7.5 |
| Adult (29-38) | 30 | 15.0 | |
| Adult (39-48) | 61 | 30.5 | |
| More than 48 | 94 | 47.0 | |
| Marital status | Married | 166 | 83.0 |
| Unmarried | 34 | 17.0 | |
| Living area | Rural | 77 | 38.5 |
| Semi Urban | 19 | 9.5 | |
| Urban | 104 | 52.0 | |
| Education | Higher Educated | 121 | 60.5 |
| Below the graduation level | 79 | 39.5 | |
| BMI-based body weight category | Normal weight | - | - |
| Overweight | 21 | 11.0 | |
| Obesity | 179 | 89.0 | |
| Triglyceride level | Normal | 58 | 29.0 |
| Boarder line high | 28 | 14.0 | |
| High | 114 | 57.0 | |
| Diabetes based on FBS | No Diabetes | 90 | 45.0 |
| Pre-Diabetes | 52 | 26.0 | |
| Diabetes | 58 | 29.0 | |
| NAFLD grading | Grade-I | 163 | 81.5 |
| Grade-II | 31 | 15.5 | |
| Grade-III | 06 | 3.0 |
A large majority (83.0%) of patients were married, while a smaller proportion (17.0%) were unmarried. Urban areas had the highest representation (52.0%), followed by rural areas (38.5%), with semi-urban areas representing just 9.5% of total NAFLD patients. 60.5% of the patients had higher education, while 39.5% had an education level below graduation. The Body Mass Index (BMI) distribution among NAFLD (Non-Alcoholic Fatty Liver Disease) patients shows 0.5% of the patients fall into the normal weight category. 10.0% of the patients are classified as overweight. 89.5% of the patients are classified as obese. Obesity is a predominant issue among this group of NAFLD patients, which is consistent with the association between obesity and the development or worsening of NAFLD. A large portion (57%) of those with NAFLD have a high triglyceride level. 29.0% of the patients have normal triglyceride levels, and 14.0% fall into the borderline high category. This highlights elevated triglycerides as a common feature in NAFLD patients, which may be associated with the risk of metabolic syndrome. Based on the assessment of fasting blood sugar monitoring, 45.0% of NAFLD patients have no diabetes. 26.0% of patients are classified as having pre-diabetes, indicating impaired fasting blood sugar levels. 29.0% of patients have diabetes, reflecting elevated fasting blood sugar levels. The patients with NAFLD were categorized into Grade-I (mild), Grade-II (moderate), and Grade-III (severe) categories, along with the corresponding percentages for each group. The majority of subjects (81.5%) have Grade-I NAFLD (mild). Grade-II (moderate) NAFLD accounts for 15.5% of the subjects. A very small proportion (3.0%) of subjects have Grade-III (severe) NAFLD.
Table 2. Anthropometric and biochemical characteristics of the female and male study subjects (N=200).
| Variables | Female (n=142) Mean (±SD) | Male (n=58) Mean (±SD) |
| Age (years) | 47.48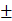 12.837 12.837 | 49.72±13.902 |
| Body mass index (kg/m2) | 32.92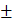 2.55 2.55 | 31.71±2.279 |
| Waist circumference (cm) | 81.86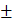 4.273 4.273 | 90.81±2.959 |
| Hip circumference (cm) | 92.76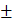 5.832 5.832 | 100.99±3.634 |
| Waist to Hip ratio | 0.88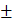 0.033 0.033 | 0.89±0.026 |
| Serum triglyceride (mg/dL) | 234.59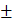 | 244.30±172.683 |
| Fasting blood sugar (mg/dL) | 109.36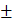 | 114.94±33.7 |
Table 2 shows anthropometric and biochemical characteristics between female and male study participants. Females have an average age of 47.48±12.837 years, while males have an average age of 49.72±13.90 years. We found that both males and females possessed higher BMI than normal. Both categories fall into obese groups. Though females have a slightly higher average BMI (32.92 kg/m²) compared to males (31.71 kg/m²). This difference suggests that females among total participants have a slightly higher level of general body fat compared to males. Waist circumference is notably larger in males (90.81±2.959) compared to females (81.86±4.273), indicating that males have more abdominal fat in this study population. We also found that hip circumference is larger in males (100.99±3.634) compared to females (92.76±5.832). The waist-to-hip ratio is slightly higher in males (0.89±0.026) compared to females (0.88±0.033).
Serum triglycerides are higher in males (234.59 mg/dL) compared to females (244.30±172.683 mg/dL). Fasting blood sugar is higher in males (114.94±33.7 mmol/L) compared to females (109.36 mmol/L). The mean blood sugar level of male participants indicates impaired blood glucose tolerance.
Table 3. Association of metabolic complications based on waist to hip ratio with female and male NAFLD patients.
| Female (n=142) | Waist to Hip Ratio Among Female | Total | p-value | ||||
0.62 | |||||||
| <=0.84 | >=0.85 | ||||||
| Grade-I | 9 | 109 | 118 | ||||
| Grade-II | 2 | 19 | 21 | ||||
| Grade-III | 1 | 2 | 3 | ||||
| Total | 12 (6%) | 130 (65%) | 142 | ||||
| Age Group | Adult (18-28) | 2 | 11 | 13 | 0.161 | ||
| Adult (29-38) | 0 | 20 | 20 | ||||
| Adult (39-48) | 7 | 40 | 47 | ||||
| More than 48 | 3 | 59 | 62 | ||||
| Total | 12 | 130 | 142 | ||||
| Male (n=58) | Waist to Hip Ratio Among Male | Total |
0.961 | ||||
| <=.89 | >=.90 | ||||||
| Grade-I | 29 | 16 | 45 | ||||
| Grade-II | 5 | 5 | 10 | ||||
| Grade-III | 2 | 1 | 3 | ||||
| Total | 36 (18%) | 22 (11%) | 58 | ||||
| Age Group | Adult (18-28) | 0 | 2 | 2 | 0.034 | ||
| Adult (29-38) | 4 | 6 | 10 | ||||
| Adult (39-48) | 12 | 2 | 14 | ||||
| More than 48 | 20 | 12 | 32 | ||||
| 36 | 22 | 58 | |||||
| Total | 200 (100%) | ||||||
Table 3 represents the association of metabolic complications based on WHR with NAFLD in female (n=142) and male (n=58) patients. Additionally, p-values are provided to assess the significance of differences. Among females, the majority (130 out of 142) have a WHR >0.85, indicating a higher risk for metabolic complications. 109 individuals with WHR >0.85 having Grade I NAFLD suggests high risk according to WHR thresholds. 19 female respondents having Grade II NAFLD and 2 having Grade III NAFLD, reflecting the metabolic complications with WHR >0.85. The p-value for NAFLD grading (0.620) suggests that the relationship between WHR and NAFLD grading is not statistically significant for females. This means that WHR may not have a strong association with the severity of NAFLD in females.
Among female NAFLD patients (n=142), a total of 13 were in the 18-28 years’ age group, with 2 having WHR ≤0.84 and 11 having WHR ≥0.85. In the 29-38 years’ group, all 20 patients had WHR ≥0.85, with none in the ≤0.84 category. In the 39-48 years’ group, 7 patients had WHR ≤0.84 and 40 had WHR ≥0.85, totaling 47. The largest group was females above 48 years, where 3 had WHR ≤0.84 and 59 had WHR ≥0.85, making a total of 62. Overall, the distribution showed increasing prevalence of higher WHR (≥0.85) with advancing age, though the association was not statistically significant (p = 0.161).
Among the 58 male NAFLD patients, the distribution of disease grading varied between those with different waist-to-hip ratios (WHR). In the group with a WHR ≥ 0.90 (n=22), Grade-I NAFLD was present in 16 patients, while Grade-II and Grade III were seen in 5 and 1 patients, respectively. In comparison, among those with a WHR ≤ 0.89 (n=36), Grade-I was observed in 29 patients, with 5 cases of Grade-II, and 2 cases of Grade-III. The p-value for NAFLD grading (0.961) indicates that WHR does not have a statistically significant impact on NAFLD grading in males.
Among the 58 male NAFLD patients, the distribution of waist-to-hip ratio (WHR) also varied notably across age groups. Of the 22 males with a WHR ≥ 0.90, the largest number (n=12) were in the more than 48 years’ age group, followed by 6 participants in the 29-38 years group, 2 in the 18-28 years group, and 2 in the 39-48 years group. In contrast, among the 36 males with a WHR ≤ 0.89, the highest number were also from the more than 48 years’ group (n=20), followed by 12 participants aged 39-48 years, 4 from the 29-38 years’ group, and none from the 18-28 years’ group. This distribution indicates that higher WHR values (≥ 0.90) are more common among older males, particularly those over the age of 48. The association between WHR and NAFLD grades in males was statistically significant (p = 0.034), suggesting a stronger link between metabolic complications and age-wise NAFLD severity.
Table 4. Correlation between different grades of NAFLD and anthropometric as well as biochemical characteristics of the patients included in this study.
| Variables | Grade-I (n=163) | Grade-II and III (n=37) | P value |
| Age (years) | 48.43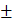 13.57 13.57 | 46.81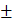 11.19 11.19 | 0.500 |
| Height (m) | 1.57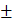 0.088 0.088 | 1.58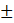 0.086 0.086 | 0.468 |
| Weight (kg) | 81.23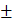 10.429 10.429 | 83.22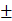 9.452 9.452 | 0.290 |
| Body mass index (kg/m²) | 32.47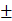 2.551 2.551 | 32.97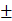 2.421 2.421 | 0.278 |
| Waist circumference (cm) | 84.43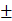 5.627 5.627 | 84.57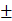 5.881 5.881 | 0.887 |
| Hip circumference (cm) | 95.04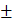 6.472 6.472 | 95.61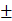 6.556 6.556 | 0.632 |
| Waist to hip ratio | 0.88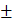 0.031 0.031 | 0.89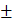 0.038 0.038 | 0.690 |
| Serum triglyceride (mg/dL) | 244.50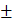 156.821 156.821 | 206.14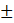 73.697 73.697 | 0.028 |
| Fasting blood sugar (mg/dL) | 111.76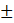 29.17 29.17 | 92.01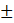 26.11 26.11 | 0.396 |
Table 4 represented the average age of Grade-I NAFLD subjects as 48.43 ± 13.57 years, while Grade-II and III NAFLD subjects had a mean age of 46.81 ± 11.19 years. The p-value (0.500) indicates that there is no significant difference in age between the two groups. The average height of Grade-I NAFLD subjects was 1.57 ± 0.088 meters, compared to 1.58 ± 0.086 meters for Grade-II and III NAFLD subjects.
The p-value (0.468) suggests no significant difference in height between the two groups. The mean weight of Grade-I subjects was 81.23 ± 10.429 kg, while Grade-II and III subjects had an average weight of 83.22 ± 9.452 kg. The p-value (0.290) indicates no significant difference in weight between the two groups. The BMI for Grade-I NAFLD subjects was 32.47 ± 2.551 kg/m², compared to 32.97 ± 2.421 kg/m² for Grade-II and III subjects. The p-value (0.278) suggests no significant difference in BMI between the two groups. The average waist circumference for Grade-I NAFLD subjects was 84.43 ± 5.627 cm, and for Grade-II and III subjects, it was 84.57 ± 5.881 cm. The p-value (0.887) indicates no significant difference in waist circumference. Hip circumference among Grade-I NAFLD subjects was 95.04 ± 6.47 cm, while in Grade-II and III subjects it was 95.61 ± 6.56 cm, showing no significant difference (p = 0.632). The waist-to-hip ratio was 0.88 ± 0.031 for Grade-I subjects and 0.89 ± 0.038 for Grade-II and III subjects. The p-value (0.690) shows no significant difference in WHR. A significant difference was observed in serum triglyceride levels between the two groups. Grade-I NAFLD subjects had a mean serum triglyceride level of 244.50 ± 156.821 mg/dl, while Grade-II and III NAFLD subjects had a mean of 206.14 ± 73.697 mg/dl. The p-value (0.028) indicates a statistically significant lower level of triglycerides in the Grade-II and III group. The fasting blood sugar level for Grade-I NAFLD subjects was 111.76± 29.17 mg/dl, and for Grade-II and III subjects, it was 92.01±26.11 mg/dl. The p-value (0.396) indicates no significant difference in fasting blood sugar levels. The results of this study indicate that there were no significant differences in most of the clinical parameters between subjects with Grade-I NAFLD and those with Grade-II and III NAFLD, including age, height, weight, BMI, waist circumference, waist-to-hip ratio, and fasting serum glucose.
However, a statistically significant difference was observed in the serum triglyceride levels, with Grade-I NAFLD subjects having significantly higher triglyceride concentrations compared to those with more advanced stages (Grade II and III) of the disease. This finding suggests that lipid metabolism might change as NAFLD progresses, and elevated triglyceride levels could be a characteristic of early-stage NAFLD.
Table 5. Binary logistic regression analysis showing the predictors of metabolic complications among NAFLD patients.
| Variables | B | S.E. | Wald | df | Sig. (p) | Exp (B) | 95% C.I. for Exp (B) |
| Waist-to-Hip Ratio (WHR | 10.563 | 3.251 | 10.57 | 1 | 0.001 | 38.56 | 4.32 - 342.68 |
| BMI (kg/m²) | 0.215 | 0.088 | 5.96 | 1 | 0.015 | 1.24 | 1.04 - 1.53 |
| FBS (mmol/L) | 0.461 | 0.172 | 7.16 | 1 | 0.007 | 1.59 | 1.14 - 2.21 |
| Triglyceride (mg/dL) | 0.004 | 0.002 | 3.96 | 1 | 0.047 | 1.00 | 1.00 - 1.01 |
| Age (years) | 0.028 | 0.017 | 2.67 | 1 | 0.102 | 1.03 | 0.99 - 1.07 |
| Sex (Male=1, Female=0) | -0.982 | 0.419 | 5.48 | 1 | 0.019 | 0.37 | 0.16 - 0.85 |
| Nagelkerke R² = 0.42, Classification Accuracy: 81.2% | |||||||
Table 5 presents the results of the binary logistic regression analysis conducted to identify factors that predict metabolic complications among NAFLD patients. The model shows that WHR, BMI, and FBS are significant predictors of metabolic complications (p < 0.05). Among them, WHR had the strongest influence (B=10.563, p=0.001), indicating that a higher WHR greatly increases the likelihood of developing metabolic complications. Similarly, BMI (B=0.215, p=0.015) and FBS (B = 0.461, p = 0.007) were also positively associated with the presence of metabolic complications, suggesting that individuals with higher BMI and blood sugar levels are more at risk. Triglyceride level (p = 0.047) showed a weak but statistically significant effect, while age (p = 0.102) was not a meaningful predictor. Sex (B = −0.982, p = 0.019) had a negative relationship, showing that males were less likely to have metabolic complications compared to females. The regression model explains about 42% of the variation in metabolic complications (Nagelkerke R² = 0.42) and correctly classifies 81% of cases. The results clearly indicate that WHR, BMI, and FBS are the most important predictors of metabolic complications among NAFLD patients, highlighting the major role of central obesity and glucose metabolism in metabolic risk.
Table 6. Ordinal logistic regression predicting NAFLD severity by clinical and biochemical variables (grade I-III).
| Predictor | B | SE | Wald χ² | p | Exp(B) | 95% CI for Exp(B) |
| Age (years) | 0.012 | 0.019 | 0.38 | 0.538 | 1.012 | 0.975, 1.051 |
| BMI (kg/m²) | 0.042 | 0.073 | 0.33 | 0.567 | 1.043 | 0.902, 1.206 |
| Waist-to-Hip Ratio | 1.486 | 1.201 | 1.53 | 0.216 | 4.418 | 0.464, 42.069 |
| Triglyceride (mg/dL) | −0.007 | 0.003 | 5.01 | 0.028 | 0.993 | 0.987, 0.999 |
| Fasting Blood Sugar (mmol/L) | 0.109 | 0.162 | 0.45 | 0.502 | 1.115 | 0.812, 1.532 |
| Gender (1 = Male) | 0.264 | 0.487 | 0.29 | 0.591 | 1.303 | 0.501, 3.388 |
Table 6 shows the results of the ordinal logistic regression used to predict NAFLD severity (Grades I-III) based on different clinical and biochemical factors. Among all the predictors, only serum triglyceride (p = .028) was found to have a significant effect on NAFLD severity. The negative coefficient (B = −0.007) means that patients with lower triglyceride levels tended to have more severe NAFLD grades. This finding is consistent with Table 4, where Grade II-III patients also had lower triglyceride levels than those with Grade-I. The WHR showed a positive but non-significant effect (B = 1.486; Exp (B) = 4.418; p = 0.216), suggesting a mild trend that higher WHR might increase the likelihood of severe NAFLD, though the result was not statistically meaningful. Other factors such as age, BMI, fasting blood sugar, and gender were not significantly related to disease severity (p > .05). The model indicates that triglyceride level is the most important factor linked to NAFLD severity, while WHR and BMI have weaker or non-significant roles in predicting disease progression among the studied patients
This study explored the association between metabolic complications and WHR among patients with NAFLD, emphasizing gender-based differences. Our findings revealed that individuals with a higher WHR were at significantly greater risk of metabolic complications, consistent with previous studies linking central obesity to NAFLD risk [24]. Similar to our results, a large-scale Japanese study demonstrated a positive association between NAFLD and increased waist-to-height ratio [24].
Previous meta-analyses have shown that NAFLD is associated with nearly a two-fold higher risk of developing type 2 diabetes and metabolic syndrome [25]. Comparable observations have been reported in various populations, including studies from Australia [26], Iran [27], and the United States [28], reported comparable findings [29] where MetS prevalence ranged between 29-34%. In Indian adults with NAFLD, the prevalence of MetS was reported to be 47% [30], highlighting its global burden.
Age-related physiological changes also contribute to metabolic disturbances, as advancing age alters metabolic regulation and increases susceptibility to NAFLD and MetS [31]. Obesity remains a predominant factor; approximately 80% of NAFLD patients in earlier reports were obese [32]. Similarly, 89.5% of participants in our study were classified as obese, reinforcing the strong link between obesity and NAFLD. These findings are consistent with Semmler et al. [33], who demonstrated strong associations between NAFLD, dyslipidemia, and hyperglycemia.
Our results also revealed that patients with Grade I NAFLD exhibited significantly higher triglyceride (TG) levels than those with more advanced stages (Grade II–III). This indicates that lipid metabolism may shift as the disease progresses and that elevated TG may be a marker of early-stage NAFLD. This observation aligns with the regression analyses, where TG levels were significantly associated with NAFLD severity. Increased triglyceride accumulation is believed to play a central role in hepatic fat deposition and the development of fatty liver abnormalities. A notable finding of our study was the higher prevalence of NAFLD and metabolic complications among females (71%) compared to males (29%). This contradicts the findings of Fattahi et al. (2016), who reported a higher prevalence in men (33.1% vs. 27.5%) [34]. The higher female prevalence in our study may be related to hormonal differences. Estrogen plays a vital role in hepatic glucose and lipid regulation; postmenopausal estrogen decline contributes to visceral fat accumulation and dyslipidemia, increasing NAFLD risk [35]. Additionally, socioeconomic and educational disparities-particularly affecting women in semi-urban and rural regions may exacerbate the risk of obesity and metabolic disorders.
Hip circumference has also been implicated as a metabolic predictor. Tekin et al. [36] reported that individuals with metabolic syndrome had larger hip circumferences compared to controls, while Dixon et al. [37] found that a smaller hip circumference was associated with dyslipidemia and MetS in obese women. Studies from Australia also documented a 21.7% prevalence of MetS with concomitant cardiovascular disease risk [38]. Moreover, the reciprocal relationship between MetS and NAFLD has been well established each condition increases the likelihood of the other [39].
Our logistic regression analysis demonstrated that WHR, BMI, and fasting blood sugar (FBS) were the strongest predictors of metabolic complications in NAFLD, whereas triglyceride level was the only biochemical marker significantly associated with disease severity. These results underscore the importance of central obesity and impaired glucose metabolism in NAFLD progression. Similar anthropometric markers (WHR and BMI) have also been validated as predictors of metabolic syndrome risk in children and adolescents [40, 41], reaffirming their clinical significance. Beyond biological and metabolic determinants, lifestyle behavior, nutritional status (poor dieting, anemia), and health awareness including parental knowledge and practices in managing diseases play vital roles in shaping disease outcomes [42, 43]. Moreover, giving emphasis on the potential of innovative educational tools such as microcredential based learning to strengthen public health capacity and promote evidence-based practice among healthcare professionals is important [44].
Despite its strengths, including gender-based analysis and integration of multiple anthropometric and biochemical variables, the study has limitations. The relatively small sample size and lower proportion of male participants may limit generalizability. Future large-scale, longitudinal studies should investigate the mechanisms underlying gender differences and the role of hormonal and genetic factors in NAFLD and its metabolic complications. Overall, our findings emphasize that WHR, BMI, FBS, and TG are critical indicators for identifying metabolic risk among NAFLD patients. Early recognition of these markers and targeted interventions focusing on obesity control, glycemic regulation, and lipid management could substantially reduce the burden of NAFLD and its associated metabolic complications in Bangladesh.
This study highlights a high prevalence of obesity and metabolic disturbances among patients with NAFLD, with a notably greater incidence of metabolic complications observed in female patients. Interestingly, although males exhibited slightly higher waist and hip circumferences, this difference may be attributed to the limited sample size and may not reflect broader population trends. The major metabolic indicators namely WHR, BMI, and FBS, emerged as significant predictors of metabolic complications. In contrast, triglyceride level was the only parameter significantly associated with the severity of NAFLD. These findings emphasize the critical role of central obesity and impaired glucose metabolism in the early detection and management of NAFLD. Moreover, it emphasized the complex and multifactorial nature of NAFLD progression, which may involve subtle metabolic alterations not fully represented by standard clinical assessments. Early detection and targeted interventions focusing on weight management, glycemic control, and lipid regulation may help prevent metabolic complications and slow disease progression. Future large-scale and longitudinal studies can validate these findings and explore the underlying biological and hormonal mechanisms contributing to gender-specific differences in NAFLD progression.
We would like to express our sincere gratitude to all the study participants for their valuable contribution and cooperation throughout this study.
This project was self-funded.
The authors declare that they don’t have any kind of conflict of interest.
This study was approved by the Ethical Review council (ERC), Memo No: BUHS/ERC/EA/24/432, Bangladesh University of Health Sciences (BUHS). The information and identity of the participants was kept private. All the Patients included in this study were informed about the nature, risk and benefit of the study. Participation in this research was fully voluntary. The respondents had remained entirely free to withdraw their participation at any stage or any time of the study. Informed written consent was taken from each patient
Original conceptualization was contributed by Sonia Akter. Data entry, study design and writing-original draft were contributed by Sonia Akter, Rahelee Zinnat, Mahbuba Khatun and Md. Afzal Hossain. Data collection was done by Sonia Akter, Md. Yousuf Hosen and Sharmin Akter. Statistical analysis and visualization were prepared by Rahelee Zinnat, Mahbuba Khatun and Shohal Hossain. Writing review was done by Fuad Hossain, Farzana Yeasmin Khusbu and Momtaz Jahan. Citation and references were prepared by Md. Afzal Hossain. All authors have read and agreed to the published version of the manuscript.
The authors declare that artificial intelligence (AI) tools were used to assist in the preparation of this manuscript (approximately 8%), specifically for improving language, grammar, and sentence structure. All content was reviewed and verified by the authors to ensure accuracy and compliance with ethical standards.