J. Biosci. Public Health. 2025; 1(3)
Health care professionals (HCPs) play a critical role in promoting public confidence in vaccination programs. However, vaccine hesitancy among HCPs during the initial rollout of COVID-19 vaccines could undermine national immunization efforts. This study assessed early acceptance and attitudes toward COVID-19 vaccination among doctors and nurses in a tertiary hospital in Dhaka, Bangladesh. A descriptive cross-sectional study was conducted from December 2020 to March 2021 among 732 HCPs recruited through purposive sampling and using a semi-structured questionnaire. Among the respondents, 76.28% were female and 79% were aged 18-50 years. Only 37.64% expressed willingness to receive the vaccine at the starting period, while 52.71% preferred to wait for post-vaccination reviews and 9.65% refused vaccination. Although 67.26% agreed or strongly agreed that vaccination is essential for COVID-19 prevention, there are concerns about safety (40.53% neutral or negative). Overall, 45.65% demonstrated satisfactory attitudes, whereas 32.06% showed dissatisfactory attitudes. Vaccine acceptance was significantly associated with sex (p = 0.032), having children (p = 0.016), and diabetes status (p = 0.041). Despite generally positive attitudes toward the importance of vaccination, more than half of HCPs exhibited hesitancy during the early phase of the vaccination program. Concerns about vaccine safety and side effects were major contributors to delayed acceptance. By revealing the factors influencing early vaccine acceptance among frontline workers, this study offers valuable insights to strengthen preparedness and enhance the effectiveness of future immunization programs such as typhoid vaccination in children.
The underlying cause of the ongoing COVID-19 pandemic is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 first appeared in Wuhan (Hubei, China) in late 2019 and quickly spread to 220 countries, posing a global threat. As of October 2025, the pandemic has inflicted a profound toll, surpassing 776 million confirmed cases and 7.1 million deaths worldwide, with enduring sequelae in health systems, economies, and social fabric [1]. The primary strategy used by most countries worldwide was to reduce the spread of infection, usually through nonpharmaceutical interventions (NPIs). These included the use of veils, hand washing, social distancing, travel restrictions, closing of schools, and partial or total lockdowns. NPIs had the ability to stop the spread of the disease up until this point, but the most promising strategy to contain the pandemic and provide motivation to lower the death and morbidity rates remains within the realm of therapeutic innovation [2-4]. Clinical innovation has seen the successful development of safe and effective antiviral treatments and vaccines. By December 2020, no antiviral drugs specifically targeting SARS-CoV-2 had been approved [5]. Vaccines, however, stand as one of the most reliable and cost-effective public health measures, saving millions of lives annually. Following the sequencing of the SARS-CoV-2 genome in early 2020 and the declaration of the pandemic by the WHO in March 2020, researchers and pharmaceutical companies raced to develop vaccines [6]. By December 19, 2020, at least 85 vaccines were in preclinical trials on animals, and 63 were in clinical trials on humans. Among these, 43 were in Phase I, 20 in Phase II, 18 in Phase III, 6 had been authorized for emergency or limited use, 2 had been approved for full use, and one vaccine had been abandoned [7]. Bangladesh launched its nationwide COVID-19 vaccination campaign in February 2021, using the Oxford University-AstraZeneca vaccine, with plans to vaccinate 3.5 million people in the first month [8]. The government aims to vaccinate 80% of its population of approximately 170 million, with each person receiving two doses four weeks apart. By February 2021, Bangladesh ranked 15th globally in terms of the number of vaccine doses administered per 100 people, with 1.26 doses given for every 100 people [9, 10]. At the initial stage of the vaccination program, the Bangladesh government did master planning to vaccinate the health workers at first, as they are working as frontline workers to fight against COVID-19. Finally, this program was completed very successfully. As per the report, vaccination milestones reflect robust primary coverage: >88% first dose and ~83% fully vaccinated against not only the HCPs but also the whole population [11]. Achieving this commendable progress in vaccination coverage was very challenging at the early phase of vaccination. It was very similar to early global evidence highlighting varying levels of vaccine hesitancy among HCPs, driven by concerns related to safety, effectiveness, rapid vaccine development, and misinformation [12].
Therefore, this study aimed to recall the scenario, including perception and willingness, and determinants of COVID-19 vaccine hesitancy of HCPs during the initial rollout phase in a public hospital in Bangladesh. This study identifies key drivers of vaccine hesitancy among health care professionals, providing essential evidence to guide targeted communication and trust-building strategies for improving uptake in future national vaccination programs.
2.1. Study Design and Participants
This descriptive cross-sectional study was carried out at a tertiary medical college hospital in Dhaka City, Bangladesh, from December 01, 2020, to March 31, 2021, with the permission of the authority. The target population was the health professionals, meaning only doctors and nurses who were working in this hospital. There were 732 health care professionals (HCP) who participated in this study. Our inclusion criteria were that the healthcare professionals, defined as only doctors and nurses in this study, were willing to participate in the study. Exclusion criteria were the professionals who were not interested in participating in the study.
2.2. Sample Size Determination
The required sample size for the study was estimated using Cochran’s formula reported elsewhere for proportion-based sampling in large populations. The formula is expressed as:
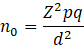
Where:
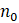 = minimum required sample size
= minimum required sample size
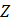 = standard normal value for a 95% confidence level (1.96)
= standard normal value for a 95% confidence level (1.96)
 = expected prevalence; 0.5 was used due to the absence of prior evidence
= expected prevalence; 0.5 was used due to the absence of prior evidence
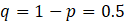
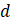 =margin of error (0.05)
=margin of error (0.05)
Substituting these values:
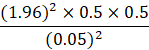
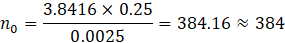
Thus, the minimum required sample size was 384 participants. However, to increase statistical power and improve the precision of estimates, a total of 732 samples were included in the final analysis, which exceeds the minimum calculated requirement.
2.3. Data Collection
Data were collected using a pretested semi-structured questionnaire. A pilot test was conducted among 5% of the sample to assess clarity and completeness. Face-to-face interviews were conducted by trained data collectors.
2.4. Statistical analysis
Data was analyzed using SPSS software based on the objectives. Finally, the data was presented in the form of a table and graph/diagram where applicable. For quality assurance, we checked data on a regular basis. The threshold for statistical significance (p-value) was considered as p ≤ 0.05, which indicates that the observed effect is statistically significant and unlikely to have occurred by chance.
The analysis of sociodemographic characters shows that 79.09% of respondents were in the age group of 18 to 50 years, and the rest, 20.91% of respondents, were aged more than 50 years (Table 1). Approximately 76.28% of respondents were female, and the rest, 23.72%, were male. Among the respondents, 38.77% were single, 49.61% were married, and the rest, 11.62%, belonged to other marital statuses. Among them, 55.63% of respondents had a child, and the rest, 44.37% of respondents, had no child. In addition, 21.65% of respondents were smokers, 12.65% of respondents were ex-smokers, and the rest, 65.70% of respondents, were non-smokers (Table 1).
Table 1. Distribution of the respondents according to their socio-demographic status.
| Characteristics | Frequency (n) | Percentage (%) |
| Age group | ||
| 18-50 years | 579 | 79.09 |
| > 50 years | 153 | 20.91 |
| Sex | ||
| Male | 174 | 23.72 |
| Female | 558 | 76.28 |
| Marital status | ||
| Single | 284 | 38.77 |
| Married | 363 | 49.61 |
Others (divorced, widowed) | 85 | 11.62 |
| Status of having children | ||
| No | 325 | 44.37 |
| Yes | 407 | 55.63 |
| Smoking status | ||
| Current smoker | 158 | 21.65 |
| Ex-smoker | 93 | 12.65 |
| Non-smoker | 481 | 65.70 |
Participants demonstrated heterogeneous attitudes toward COVID-19 vaccination. As shown in Table 2, a strong majority (67.2%) agreed or strongly agreed that vaccination is important for protection against COVID-19, while only 12.6% expressed strong disagreement. Similarly, 63.4% believed that pharmaceutical companies can produce safe and effective vaccines. However, 40.4% remained neutral, skeptical, or strongly disagreed regarding vaccine safety in Bangladesh, and 46.5% reported worry or high concern about vaccination. Notably, 32.9% held a perception that vaccines manufactured in Europe or America are safer than vaccines from other countries. Distrust toward nationally recommended or available vaccines was substantial (39.0% agree/strongly agree), and nearly 41.8% explicitly rejected the notion that vaccines were safe, either by agreeing that vaccines in Bangladesh are unsafe or expressing direct distrust. Concerns about side effects were prominent, with 50.5% agreeing or strongly agreeing that fear of side effects could deter vaccine uptake. Approximately 51.0% also anticipated high refusal rates among the general population even after governmental vaccine licensing. Support for universal free vaccine access was mixed, with only 37.8% agreeing or strongly agreeing, while 41.2% disagreed or strongly disagreed.
Table 2. Attitudes of participants toward vaccination-related statements.
| Statements | Response of the Participants | |||||||||
| Strongly Disagree | Disagree | Neutral | Agree | Strongly Agree | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| It is important to get a vaccine to protect the people from COVID-19. | 38 | 5.2 | 54 | 7.4 | 147 | 20.1 | 160 | 21.8 | 332 | 45.4 |
| Pharmaceutical companies are going to develop safe and effective COVID-19 vaccines. | 36 | 4.9 | 60 | 8.2 | 171 | 23.4 | 159 | 21.7 | 305 | 41.7 |
| COVID-19 vaccines made in Europe or America are safer than those made in other world countries. | 94 | 12.8 | 148 | 20.2 | 255 | 34.8 | 110 | 14.9 | 125 | 17.1 |
| Side effects will prevent me from taking a vaccine for the prevention of COVID-19. | 93 | 12.6 | 95 | 13.0 | 174 | 23.7 | 159 | 21.7 | 211 | 28.8 |
| Most people will refuse to take the COVID-19 vaccine once licensed in Bangladesh. | 84 | 11.4 | 101 | 13.8 | 173 | 23.6 | 166 | 22.6 | 209 | 28.4 |
| The government will make the vaccine available for all citizens for free. | 124 | 16.9 | 148 | 20.1 | 183 | 25.0 | 123 | 16.8 | 154 | 21.0 |
| I do not believe that vaccine is safe. | 154 | 21.0 | 153 | 20.8 | 128 | 17.5 | 158 | 21.6 | 138 | 18.8 |
| I am worried about COVID-19 vaccination. | 153 | 20.8 | 152 | 20.7 | 155 | 21.2 | 129 | 17.6 | 143 | 19.5 |
| I am worried about the side effects of the COVID-19 vaccination. | 144 | 19.7 | 175 | 23.8 | 125 | 17.0 | 157 | 21.4 | 131 | 17.8 |
| I do not trust the recommended and available vaccine in our country. | 174 | 23.7 | 167 | 22.8 | 120 | 16.3 | 149 | 20.3 | 122 | 16.6 |
The findings in Table 3 illustrate a generally favorable attitude toward COVID-19 vaccination among respondents, with nearly half expressing positive sentiments. However, a notable proportion (approximately one-third) held negative attitudes, highlighting persistent concerns or hesitancy. The distribution of respondents’ attitudes toward COVID-19 vaccination showed that 45.7% (n = 335) had a positive attitude (satisfactory or very satisfactory), 32.1% (n = 234) had a negative attitude (dissatisfactory or very dissatisfactory), and 22.3% (n = 163) were neutral. The highest proportion of respondents (25.6%) reported a very satisfactory attitude toward vaccination, while the lowest proportion (14.9%) reported a very dissatisfactory attitude.
Table 3. Level of attitude of the respondents towards COVID-19 vaccination.
| Level of attitude | Frequency (n) | Percentage (%) |
| Very dissatisfactory | 109 | 14.94 |
| Dissatisfactory | 125 | 17.12 |
| Neither satisfactory nor dissatisfactory | 163 | 22.29 |
| Satisfactory | 147 | 20.09 |
| Very satisfactory | 188 | 25.56 |
| Total | 732 | 100.00 |
The analysis of respondents’ willingness to receive the COVID-19 vaccine revealed a clear pattern of early-phase hesitancy among health care professionals. As illustrated in Figure 1, only 37.64% of participants reported immediate readiness to take the vaccine. More than half (52.71%) preferred to delay vaccination until observing others’ experiences, reflecting substantial caution during the initial rollout period. Around 9.65% refuse to receive vaccine in early phase. Furthermore, when asked whether they would recommend vaccination to their close contacts, 47.76% stated that they would advise friends and family to get vaccinated, whereas the remaining 52.24% indicated that they would not provide such recommendations (data not shown here). This discrepancy between personal willingness and advisory behavior suggests additional layers of uncertainty and incomplete confidence in the newly introduced vaccines.
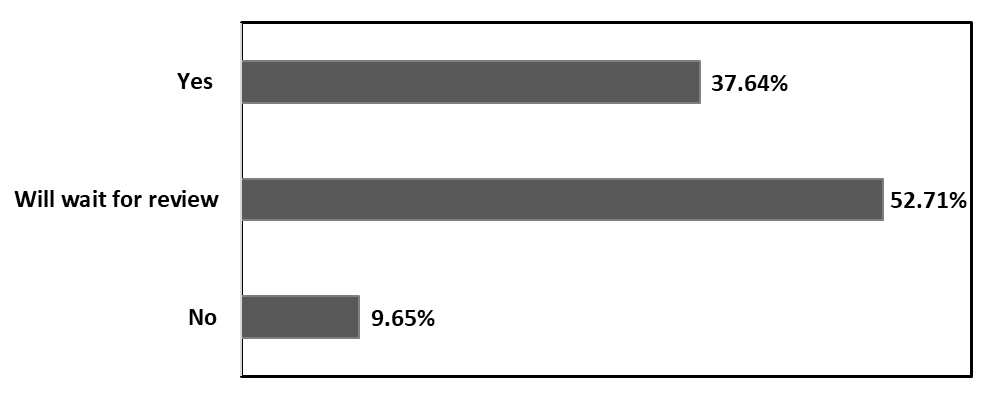
Figure 1. Respondent’s willingness to take COVID-19 vaccine.
Further analysis examined the influence of comorbid conditions on the willingness of health care professionals to receive early-stage COVID-19 vaccination, as illustrated in Figure 2. The distribution of respondents by medical history. Notably, 82.2% of respondents without chronic disease and 87.6% who did not receive an influenza vaccine last year were included in the study. Among respondents with diabetes and hypertension, 60.3% and 45.7%, respectively, were willing to take the COVID-19 vaccine, indicating higher acceptance among those with certain comorbidities.
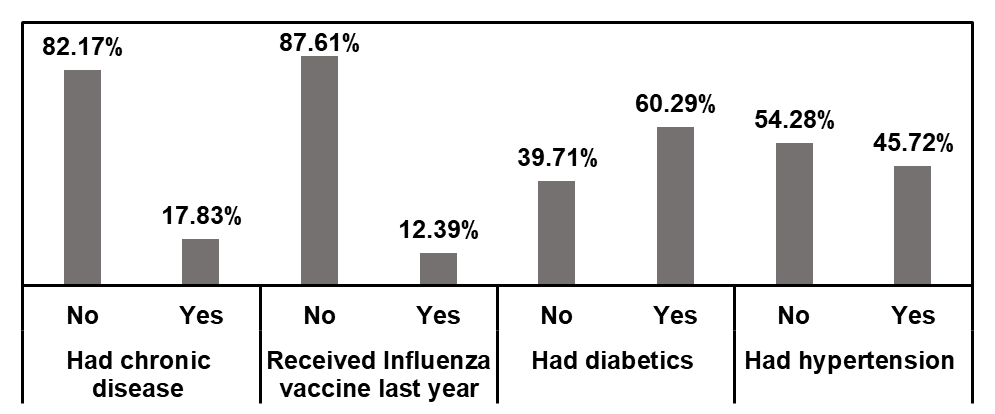
Figure 2. Distribution of the respondents according to their medical history.
Table 4 presents the association between respondents’ socio-demographic and medical characteristics and their willingness to accept the COVID-19 vaccine during the early phase of rollout. Vaccine acceptance was higher among females (52/558, 9.3%) compared to males (19/174, 10.9%), with a statistically significant association (P = 0.032). Respondents without children were significantly more willing to take the vaccine compared to those with children (P = 0.016). Among medical conditions, individuals with diabetes showed a significantly higher acceptance of the vaccine (43/441) than those without (28/291; P = 0.041). Other factors, including age, marital status, smoking, chronic disease, influenza vaccination, and hypertension, were not significantly associated with vaccine acceptance (P > 0.05 for all).
Table 4. Association of the acceptance of vaccine with the socio-demographic characteristics and medical history of the respondents.
Variables | Frequency | Acceptance/ Willing to take COVID-19 Vaccine | P value | ||
| Yes | Wait for review | No | |||
| Socio-demographic characteristics | |||||
| Age group | |||||
| 18-50 years | 579 | 63 | 327 | 189 | 0.067 |
| > 50 years | 153 | 8 | 59 | 86 | |
| Sex | |||||
| Female | 558 | 52 | 317 | 189 | 0.032 |
| Male | 174 | 19 | 69 | 86 | |
| Marital status | |||||
| Single | 284 | 25 | 156 | 103 | 0.071 |
| Married | 363 | 39 | 189 | 135 | |
| Others | 85 | 7 | 41 | 37 | |
Status of having children | |||||
| No | 325 | 32 | 176 | 117 | 0.016 |
| Yes | 407 | 39 | 210 | 158 | |
| Smoking status | |||||
| Current smoker | 158 | 19 | 81 | 58 | 0.088 |
| Ex-smoker | 93 | 7 | 46 | 40 | |
| Non-smoker | 481 | 45 | 259 | 177 | |
| Medical history | |||||
| Had chronic disease | |||||
| No | 601 | 52 | 321 | 228 | 0.092 |
| Yes | 131 | 19 | 65 | 47 | |
| Received Influenza vaccine last year | |||||
| No | 641 | 62 | 334 | 245 | 0.059 |
| Yes | 91 | 9 | 52 | 30 | |
| Had diabetics | |||||
| No | 291 | 28 | 151 | 112 | 0.041 |
| Yes | 441 | 43 | 235 | 163 | |
| Had hypertension | |||||
| No | 397 | 37 | 211 | 149 | 0.076 |
| Yes | 335 | 34 | 175 | 126 | |
The rationale of our study was to evaluate the acceptance and attitude towards COVID-19 vaccine at very early phase among the health care professional of a selected tertiary level hospital at Dhaka, Bangladesh. The low initial acceptance of COVID-19 vaccine among HCWs could also have broader consequences.
This hesitancy, characterized by a substantial proportion opting to delay vaccination pending peer reviews, aligns with global patterns where concerns over safety, efficacy, and rapid development timelines have impeded early adoption [12]. Such reluctance not only delays herd immunity but also amplifies broader societal impacts, as vaccinated HCPs are more inclined to endorse vaccines to their networks, thereby influencing patient and community behaviors. Studies have shown that HCWs who are vaccinated are more likely to recommend vaccines to friends, family, and their patients [13-15].
Attitudes toward vaccinations were clearly divergent. While many participants acknowledged its significance in disease prevention, a substantial number continued to be concerned about vaccine safety, side effects, and trust. Similar results have been seen in various low- and middle-income nations, where the majority of people think vaccines help prevent disease, whereas only a tiny percentage of people concern of its side effects [16-18]. Similarly, at the beginning of COVID-19 vaccination program, some people from different professions in Bangladesh have also questioned about the government's free vaccination program, its effectiveness which may because of disinformation or prior distrust of public health initiatives by Government [19, 20]. This highlights the important role of public awareness campaign and trust-building initiatives to mitigate vaccine-related anxiety, particularly in situations with limited resources.
The findings highlight that vaccine acceptance is influenced by both socio-demographic and health-related factors. This study highlights key determinants of COVID-19 vaccine acceptance in the surveyed population. Female respondents and individuals without children demonstrated greater willingness to accept vaccination, suggesting that perceptions of risk and responsibility toward family may influence vaccination behavior [12]. Interestingly, participants with diabetes exhibited higher acceptance, potentially reflecting increased awareness of COVID-19 risks among those with comorbidities. The lack of significant associations with age, marital status, smoking, chronic disease, influenza vaccination history, and hypertension aligns with previous studies indicating that socio-demographic and medical factors can have heterogeneous effects on vaccine attitudes [21]. These associations emphasize the role of tailored interventions, such as enhanced engagement between HCPs, policymakers, and health authorities to co-develop recommendations, thereby rebuilding trust [22]. Consistent with our findings, integrating microcredentials into public health training may enhance vaccine confidence and communication skills among frontline workers, supporting future immunization readiness in Bangladesh [23]. Moreover, recognizing COVID-19's zoonotic origins underscores the applicability of a One Health framework in addressing vaccine hesitancy, integrating human, animal, and environmental health perspectives to mitigate future pandemics [24].
Despite the findings, the study's cross-sectional methodology limitations the capacity to draw conclusions about correlation, and its dependence on a single urban hospital with non-random sample limits its applicability to various kinds of Bangladeshi contexts. In order to monitor changing attitudes and assess interventions supposed to mitigate hesitation, future research should use longitudinal methods and larger sampling, thereby strengthening preparedness for forthcoming immunization campaigns.
The study revealed mixed responses regarding COVID-19 vaccine uptake among the 732 health care professionals. Although 37.64% expressed immediate willingness to receive the vaccine, more than half (52.71%) preferred to wait for others’ experiences before deciding, indicating notable uncertainty and cautiousness. A smaller proportion (9.65%) reported refusal to vaccinate. Attitude assessment further demonstrated diverse perceptions: 32.06% held dissatisfactory attitudes (very dissatisfactory or dissatisfactory), 22.29% remained neutral, whereas 45.65% reported satisfactory to very satisfactory attitudes toward COVID-19 vaccination. These findings reflect divided opinions and varying levels of confidence among frontline workers despite their critical role in promoting immunization. Addressing vaccine hesitancy among health care professionals is essential to improving overall acceptance, as their choices influence public trust and uptake. Targeted interventions are needed to address concerns related to vaccine safety and effectiveness, improve awareness, and build institutional trust. Future studies should explore determinants of hesitancy in greater depth and assess how evidence-based communication strategies can enhance vaccine confidence among both health workers and the wider population.
The authors express their sincere gratitude to the administrative authority of hospital for granting permission to conduct this study. We are especially thankful to all participating physicians and nurses for their cooperation and valuable responses during data collection. The authors acknowledge the academic and ethical support provided by the Faculty of Health and Life Sciences, Daffodil International University. Finally, the authors are grateful to everyone who contributed directly or indirectly to the successful completion of this research.
This research did not receive any grant to carry out this study.
The authors declare no conflicts of interest.
Verbal consent was taken from the study participants. The Ethics Review Committee of Faculty of Health and Life Sciences of Daffodil International University (Ref: REC/FAHS/2020/25) approved the study protocol and the issue of ethical clearance. All data was kept confidential by the principal investigator. The anonymity and privacy were maintained strictly.
The authors disclose that artificial intelligence (AI) tools were used for improving language, grammar, and formatting which is about 8%. All content was verified by the authors to ensure accuracy and compliance with ethical standards. The authors shall be solely responsible for any misconduct related to this work.