J. Biosci. Public Health. 2025; 1(2)
Meat remains a vital source of protein globally, yet its microbial safety is often compromised in low-resource settings due to unhygienic handling and lack of regulatory oversight. This study aimed to assess the bacterial contamination in local meat shops (chopping board, cleaning drum, utensils) and evaluate antibiotic resistance patterns of isolated bacteria. Moreover, a cross-sectional survey involving 224 meat sellers was conducted to assess hygiene practices and food safety knowledge. A total of 45 samples were collected from beef and chicken cutting boards and dressing drums across various retail markets. Bacterial identification was conducted through culture and biochemical analysis, while antibiotic susceptibility was determined using the Kirby-Bauer disc diffusion method. Among the 45 samples, 27 (60%) showed bacterial growth, with Escherichia coli (37.04%), Pseudomonas spp. (22.22%) and Klebsiella spp. (14.81%) identified as the most prevalent bacteria. Alarmingly, high resistance was observed against azithromycin (70.37%), tetracycline (63%), and ciprofloxacin (63%), indicating widespread antimicrobial resistance. Survey results revealed limited food safety awareness, with 88.4% of sellers lacking any formal training and over 50% never using protective gloves, aprons. The above findings demonstrate the importance of improved hygienic operations, legal enforcement and public health initiatives are needed to reduce microbiological hazards and manage antibiotic resistance in Bangladesh's local meat retail sector.
Meat is the major source of protein and valuable qualities of vitamins for most people in many parts of the world and is essential for the growth, repair and maintenance of body cells and necessary for our everyday activities [1]. It is pertinent to mention that about 30% of zinc in our diet comes from meat and meat products [2]. Poultry meat is gaining popularity in most countries, including Bangladesh due to its low cost. Poultry and livestock contribute ~ 1.85% of the total GDP of the national economy of Bangladesh [3]. Bangladesh’s livestock is divided into two main species: ruminants and poultry. Cattle, buffaloes, sheep, and goats are under Ruminants and ducks and chickens are under Poultry. The meat products are frequently contaminated with different bacterial hazards, which can be transmitted to humans [4]. The microbial load in meat products can be significantly reduced through proper processing. However, contamination often begins at the slaughtering stage and continues during handling, storage and retail. Spoilage organisms like Pseudomonas spp. can proliferate rapidly, rendering meat unfit for consumption. Pathogenic bacteria such as Aeromonas hydrophilia, Escherichia coli, and Staphylococcus aureus can cause foodborne illnesses through infection, intoxication, or toxic-infection which poses serious public health risks [2]. Contamination may originate from unsanitary abattoirs, contaminated equipment, handlers’ poor hygiene or environmental sources such as air and water [5, 6]. The hygienic quality of meat is a critical determinant of its microbiological safety as improper food handling further increases the risk of contamination [7]. Alarmingly, many of these pathogens have been reported as multidrug-resistant (MDR), intensifying concerns regarding food safety and public health. Despite these risks, there is a lack of comprehensive market-wise data on meat safety in Bangladesh.
Aside from that, antibiotic resistance is one of the biggest threats to global health, food security, and development today. Food contamination with antibiotic-resistant bacteria can also be a major threat to public health [8, 9]. This is because antibiotic resistance determinants can be transferred to other pathogenic bacteria. Global drug resistance is influenced by various factors. These includes overpopulation, greater international travel, increased use of antibiotics in hospitals. Other factors are livestock production, selection pressure, poor sanitation, wildlife dispersal, and a subpar sewage disposal system [10, 11]. Antibiotic therapy is one of the most commonly used methods of infection control in modern medicine.
Poultry and cattle farmers frequently use antibiotics to promote rapid growth in chickens and cows. These antibiotics can subsequently enter the environment, contaminating water and soil. When such antibiotics are later administered to humans or animals, they may be ineffective because microorganisms develop resistance [12]. Antibiotic resistance in foodborne pathogens is sometimes harder to treat whereas antibiotic susceptibility can facilitate recovery. However, many life-saving antibiotics are becoming increasingly ineffective due to their unnecessary use and overuse. It has been reported that up to 67% of commonly available and widely used antibiotics are no longer functioning effectively in the human body [13]. Antimicrobial resistance (AMR) is an escalating global threat affecting the health of humans, animals and the environment. Multidrug-resistant (MDR) bacteria, commonly referred to as superbugs have emerged, spread, and become persistent in various ecosystems [14]. These MDR bacteria can be found across human, animal, and environmental interfaces, forming interconnected transmission pathways. Several factors contribute AMR crisis. These include the excessive use of antibiotics in livestock, pets, and aquaculture, the availability of antibiotics without prescriptions, increased global travel, poor sanitation and hygiene. Others are the discharge of unmetabolized antibiotics or their residues into the environment through manure [15]. However, literature on the microbial status of all kinds of meat processing centers, retail sellers, possible microbial infections from these sources and the development of antibiotic resistance among the consumers as well as sellers are still limited in Bangladesh [16].
Therefore, the main aim of this study was to evaluate the microbiological quality of meat-cutting surfaces in Dhaka’s retail markets, characterize the antibiotic resistance profiles of isolated bacteria, and assess the hygiene practices and food safety knowledge of meat sellers. By identifying contamination hotspots and resistance patterns, this research seeks to inform evidence-based interventions to enhance food safety and mitigate the public health threat posed by AMR in the meat retail sector.
2.1. Study Design and Collection of Samples
This study involved the collection and analysis of a total of 45 samples from three different sources (15 from each category), such as (i) beef, meat-cutting wooden boards and utensils, (ii) chicken dressing drums, and (iii) chicken cutting wooden boards and utensils from local meat retail shops in different zones across Dhaka and nearby markets and meat shops. At least 2 (two) samples from each spot of meat retail shops were collected for microbial quality analysis in the laboratory. After collection, the samples were sealed and securely packed in biohazard bags immediately to avoid environmental contamination and properly labelled. The samples were taken to the lab with proper precautions. In the laboratory, to explore the existence of any microorganism, especially bacteria, all samples were examined sequentially. Following a series of biochemical tests, all bacteria were then isolated and thoroughly characterized. In addition, the multidrug resistance capability of the identified bacteria was also examined.
2.2. Cross-Sectional Survey
In addition to evaluate the knowledge on microbial contamination of meat and daily practices by the meat sellers, a small survey was conducted among the meat sellers (n=224). During the collection of samples for laboratory tests, a brief structured questionnaire was used to collect the responses from the meat sellers with their permission. Irregular sellers and age of business less than one year in this profession were excluded from this survey. Data analysis for this study was conducted using Microsoft Excel and IBM SPSS Statistics, Version 25.0 (Chicago, IL, USA). Descriptive statistics, such as frequencies, percentages, means, and standard deviations, were calculated. Knowledge Level Scoring System: A scoring system was developed to assess respondent’s knowledge levels related to contamination of meat and health risk. All participants were asked to respond to five structured questions, with a total possible knowledge score of 6. The questions were as follows: (i) Raw meat can contain germs; (ii) Raw meat can be contaminated through air, mosquitoes, and flies; (iii) If sellers wear unsensitized cloth and use knives/choppers/cutting boards, it can contaminate the meat; (iv) Germs from raw meat can spread to customers and contaminate other foods, especially when raw meat is stored in the refrigerator alongside items like fruits and vegetables and (v) Government authorities should arrange training/workshops for retail meat sellers. The correct answer score is 1, and incorrect answers receive a ‘0’ score. The score obtained by a participant 4 and above (5) means “good knowledge,” while a score of 3 is “average knowledge” and below 3 (1 and 2) means “poor knowledge.”
2.3. Chemicals and Reagents
Blood Agar Base (40.0 g/L), MacConkey Agar (51.53 g/L), Mueller Hinton Agar (38.0 g/L), Kligler Iron Agar (57.52 g/L), MIU Medium Base (18.0 g/L), 40% Urea Solution, Kovacs’ Indole Reagent, and antibiotic discs for sensitivity testing were purchased from HiMedia Laboratories Pvt. Ltd., India. Crystal violet, Gram's iodine, and carbol fuchsin were obtained from DLC, Bangladesh. 95% ethanol was purchased from SUPELCO, UK. Hydrogen peroxide, oxidase reagent, cotton, hand gloves, distilled water, a test tube, a Petri dish, a platinum wire loop, and a swab stick (for sample collection) were collected from local suppliers in Bangladesh. Commercial antibiotic discs containing specific concentrations of amoxyclav (amoxicillin-clavulanic acid), azithromycin, cefixime, ciprofloxacin, cefepime, cefuroxime, and tetracycline for antibiotic sensitivity tests were collected from the available local market and used in this study.
2.4. Preparation of Media and Culture of Meat Samples
Blood agar (40.0 g/L), MacConkey agar (51.53 g/L), and Mueller Hinton agar (38.0 g/L) were used for the culture and isolation of bacteria by streak-plate culture technique by following appropriate laboratory procedures. At first Agar Powder was measured and taken into a conical flask containing distilled water. Then heated in an autoclave at 121°C for 15 minutes and mixed the media with gentle swirling and allowed to completely dissolve. Before pouring into the sterile petri dishes, it was cooled to room temperature. To prepare blood agar media 5% v/v sterile defibrinated sheep blood was added at the temperature of 45-50°C and then poured into the sterile Petri dish. A total of 45 samples were tested for microbial analysis using three selective media. Each sample was streaked on selected media using a sterile platinum wire loop by the streak plate method. Then all the plates were incubated for 24 hours at 37°C.
2.5. Biochemical Tests
The selected bacterial strains were differentiated based on their metabolic patterns using different biochemical tests, including the Gram staining, oxidase, Kligler Iron Agar (KIA) test, catalase test, motility etc.
2.6. Gram Staining
Gram’s iodine and crystal violet were used as the primary and moderate stains to perform this test. 95% ethanol and safranin were used for decolorization and counterstaining, respectively. The result was interpreted according to the purple color for gram-positive and red color for gram-negative bacterial isolation using a light microscope with oil immersion.
2.7. Oxidase Test
To perform this test, a freshly made 1% solution of N, N, N, N-tetramethyl-p-phenylenediamine dihydrochloride in water was soaked on wet filter paper. Then, using a platinum wire loop, a small amount of bacterial growth was rubbed on it. The immediate development of deep purple color indicates the oxidase test is positive.
2.8. Motility Test
The motility test is used to determine the motility of an organism. Motile organisms contain flagella, which facilitate movement beyond the point of inoculation. Most of the motile. Bacteria are generally bacilli, except for a few motile cocci. A small aliquot of a cultured bacterial colony was taken from the petri dish and placed into the cavity slide with 1 drop of saline. A thin smear was created and was ready to be observed through the electronic microscope with a 40X objective to check out the motility.
2.9. Catalase Test
Pick up the test colony on a platinum loop and immerse it in a few drops of 3% H₂O₂ (hydrogen peroxide). Rapid bubbling indicates oxygen production and a positive test. This test is used to Differentiate between staphylococci (catalase positive) and streptococci (catalase negative).
2.10. Colony Count
The plate count method was to count the single colony-forming unit (CFU) of bacteria. To perform this test, a 10 μL sample was taken, and the serial dilution technique was applied up to the dilution factor of 10^5 for forming a single colony. Then the plates containing the bacteria were incubated for 24 hours at 37°C. Finally, individual colonies were counted from these plates containing bacterial colonies.
2.11. Antibiotic Sensitivity Test
To identify the in vitro susceptibility of isolated bacteria to various antimicrobial drugs, the Kirby-Bauer method [17] was used by utilizing antibiotic disc diffusion on Muller-Hinton agar (38.0 g/L). This method allowed for the assessment of the antibiotic effectiveness, demonstrating the pathogen’s inhibition to a degree proportionate to the diameter of the zone of inhibition produced by the antimicrobial’s diffusion encircling the disc onto the agar medium. Commercial antibiotic discs containing AMC=amoxicillin (10 μg), AZM=azithromycin (15 μg), CFM=cefixime (5 μg), CIP=ciprofloxacin (5 μg), CXM=cefuroxime (30 μg), and TE=Tetracycline (30 μg) were used in this study. The isolated bacterium was swabbed on the Mueller Hinton agar media, and the antibiotic discs were placed on top and incubated in the media overnight at 37°C. If the organism is killed or inhibited by the concentration of the antibiotic, there will be a clear zone in the immediate area around the disc: This is called the zone of inhibition. The zone sizes are looked up on a standardized chart to give a result of Sensitive (S), Resistant (R), or Intermediate (I).
3.1. Analysis, Identification, and Characterization of Bacterial Sample
In this study, a total of 45 wooden meat cutting board samples (15 × 3 = 45) were collected from three distinct categories-beef cutting boards, chicken dressing drums, and chicken cutting boards-across 15 different meat retail shops in Dhaka, Bangladesh (Figure1). Immediately after collection, the samples were aseptically sealed in sterile, labeled biohazard bags to prevent cross-contamination and transported in insulated coolers maintained at 4 °C to the laboratory. Upon arrival, the samples were stored at −20 °C until further microbiological analysis to ensure microbial stability and prevent degradation.

Figure 1. Representative photographs of the sources of sample collection from meat shops, including wooden cutting boards, chicken dressing drums (panel A), and beef cutting boards with utensils (panel B).
Three selective media-MacConkey Agar (51.53 g/L for Gram-negative selection), Mueller-Hinton Agar (38.0 g/L for antibiotic susceptibility testing), and Blood Agar (40.0 g/L for general growth)-are used to cultivate bacteria, as shown in Figure 2. The streak-plate method was used to isolate bacteria in an aseptic setting, and the bacteria were incubated for 24 to 48 hours at 37°C. Heterogeneous microbial contamination was confirmed by distinct colonial morphologies that were seen in samples from chicken cutting boards, beef cutting boards, and chicken dressing drums. Viable counts were further measured using the spread-plate technique, where variations in growth density corresponded to variations in the microbial load across sample categories.
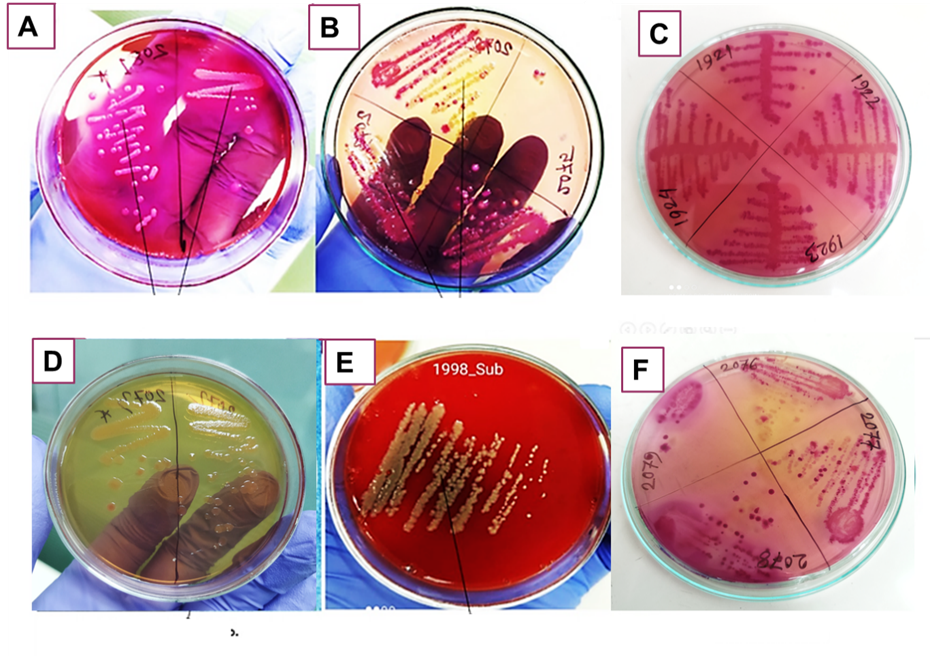
Figure 2. Bacterial growth patterns observed on samples collected from different local meat retail shops after cultivation using the spread-plate technique. Here, (A) Escherichia coli, (B) Pseudomonas spp., (C) Klebsiella spp., (D) Enterobacter spp., (E) Staphylococcus aureus. and (F) Citrobacter spp.
Table 1 shows the summary results of biochemical tests of 27 samples where bacteria were grown among the total of 45 samples, which were collected from three sources: Beef Cutting Wood (n=15), Chicken Drum (n=15), and Chicken Cutting Wood (n=15). After characterization, six different kinds of bacteria were identified among the 27 growth positive samples, including Escherichia coli, Pseudomonas spp., Citrobacter spp., Klebsiella spp., Enterobacter spp., and Staphylococcus aureus. Among the 27 samples analyzed, Escherichia coli was the most frequently identified bacterium, present in 10 samples (37.04%), followed by Pseudomonas spp. in 6 samples (22.22%) and Klebsiella spp. in 4 samples (14.81%). Both Staphylococcus aureus and Enterobacter spp. were found in 2 samples each (7.41%), as was Citrobacter spp., also present in 2 samples (7.41%). These findings highlight Escherichia coli as the predominant isolate among the tested bacterial species. All isolated organisms underwent standard biochemical testing to determine their physiological and metabolic characteristics. In addition, out of 15 samples, 12 samples collected from chicken chopping boards/utensils show positive growth of bacteria. This means chicken shops are more vulnerable to microbial contamination.
Table 1. Results of biochemical analyses indicate the presence of diverse microbial species on beef and chicken cutting boards, as well as chicken cleaning drums.
| Sl. | Sample source | Bacteria Identified | Gram staining | Motility test | Oxidase test | Catalase test | Colony Count (CFU) |
| 1 | Beef chopping board/utensils
| Enterobacter spp. | - | + | + | + | 1x10^3 |
| 2 | Pseudomonas spp. | - | + | + | + | 1x10^4 | |
| 3 | Pseudomonas spp. | - | + | + | + | 1x10^6 | |
| 4 | Pseudomonas spp. | - | + | + | + | 1x10^4 | |
| 5 | Klebsiella spp. | - | - | - | + | 1x10^6 | |
| 6 | Staphylococcus aureus | + | + | + | + | 1x10^5 | |
| 7 | Citrobacter spp. | - | + | - | + | 1x10^5 | |
| 8 | Escherichia coli. | - | + | - | + | 1x10^5 | |
| 9 | Klebsiella spp. | - | - | - | + | 1x10^5 | |
| 10 | Chicken chopping board/utensils
| Klebsiella spp. | - | - | - | + | 1x10^4 |
| 11 | Escherichia coli | - | + | - | + | 1x10^4 | |
| 12 | Escherichia coli. | - | - | - | + | 1x10^3 | |
| 13 | Escherichia coli. | - | + | - | + | 1x10^6 | |
| 14 | Klebsiella spp. | - | - | - | + | 1x10^5 | |
| 15 | Pseudomonas spp. | - | + | + | + | 1x10^3 | |
| 16 | Escherichia coli. | - | + | - | + | 1x10^5 | |
| 17 | Escherichia coli. | - | + | + | + | 1x10^3 | |
| 18 | Escherichia coli. | - | + | - | + | 1x10^5 | |
| 19 | Escherichia coli. | - | + | - | + | 1x10^5 | |
| 20 | Enterobacter spp. | - | + | + | + | 1x10^4 | |
| 21 | Escherichia coli. | - | + | - | + | 1x10^6 | |
| 22 | Chicken dressing drum
| Staphylococcus aureus. | + | + | + | + | 1x10^6 |
| 23 | Escherichia coli. | - | + | - | + | 1x10^4 | |
| 24 | Pseudomonas spp. | - | + | + | + | 1x10^5 | |
| 25 | Pseudomonas spp. | - | + | + | + | 1x10^5 | |
| 26 | Citrobacter spp. | - | + | - | + | 1x10^3 | |
| 27 | Escherichia coli. | - | + | - | + | 1x10^4 |
Tests performed included Gram staining, motility, oxidase, catalase, and coagulase tests. Colony count estimations were also performed to assess bacterial load. The results revealed specific patterns consistent with established characteristics of each species. Escherichia coli. was found mostly in the chicken cutting wood. All isolated bacteria were identified as Gram-negative, except Staphylococcus aureus and showed positive result in catalase test. In the case of the oxidase test, about half (50%) of the isolated bacteria showed positive result. Klebsiella spp. was found to be non-motile, whereas others were found as motile. A profuse amount of Pseudomonas spp. bacteria was found in the beef cutting wood which is also an alarming condition. In chicken drum, Escherichia coli. and Staphylococcus aureus. were found at a profuse level. After the isolation and characterization, antibiotic sensitivity and resistance profiling were carried out for all bacteria of 27 samples as summarized in the next section.
3.2. Antibiotic Resistance Profile
To evaluate the antibiotic resistance profiles of bacteria isolated from various meat retail shops, the zone of inhibition method was employed as a rapid, qualitative screening tool to assess the efficacy of selected antimicrobial agents. Antibiotic susceptibility testing was conducted using six antibiotics: Amoxicillin-Clavulanic Acid (AMC), Azithromycin (AZM), Cefixime (CFM), Ciprofloxacin (CIP), Cefuroxime (CXM), and Tetracycline (TE). The results revealed a high level of resistance across all isolated bacterial strains to these antibiotics.
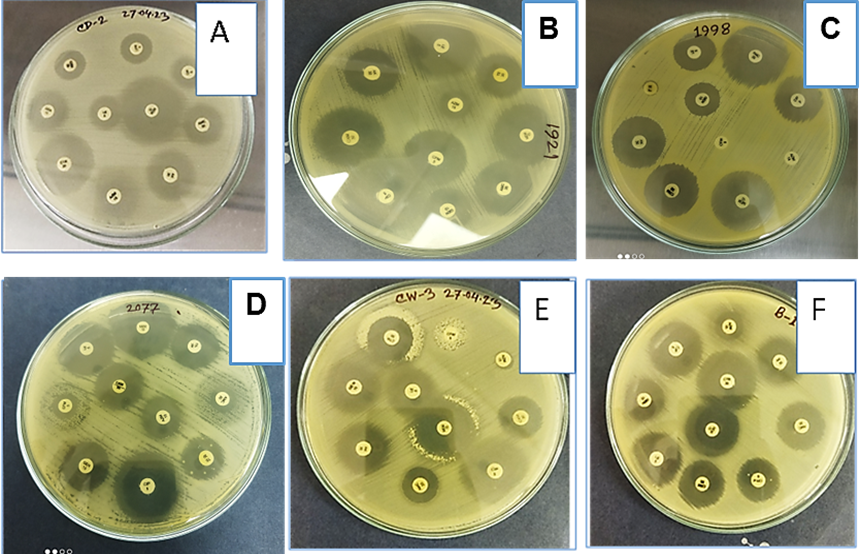
Figure 3. Representative images of antibiotic sensitivity test. Different antibiotic discs were placed on Muller-Hinton agar media and cultured for bacterial growth. (A) Escherichia coli, (B) Pseudomonas spp., (C) Citrobacter spp., (D) Klebsiella spp., (E) Enterobacter spp., and (F) Staphylococcus aureus using different kinds of antibiotics.
As shown in Figure 3, the effectiveness of each antibiotic was determined by the size of the inhibition zone produced by the diffusion of the antimicrobial agent into the agar medium surrounding the disc. A larger zone indicated greater bacterial susceptibility, whereas the absence of an inhibition zone suggested that the antibiotic was ineffective in inhibiting bacterial growth.
Table 2. Summary of antibiotic resistance profiles against the isolated bacteria. Here, S stands for sensitivity (> 17 mm) and R for resistance (<14 mm) based on the calculated zone of inhibition.
| Sl. No. | Sample source | Bacteria Identified | AMC | AZM | CFM | CXM | CIP | TE |
| 1 |
Beef chopping board/utensils
| Enterobacter spp. | S | R | S | S | S | S |
| 2 | Pseudomonas spp. | S | R | S | S | S | S | |
| 3 | Pseudomonas spp. | R | R | R | R | S | R | |
| 4 | Pseudomonas spp. | R | R | S | S | S | R | |
| 5 | Klebsiella spp. | S | R | R | R | S | R | |
| 6 | Staphylococcus aureus | S | R | R | S | R | R | |
| 7 | Citrobacter spp. | S | R | R | S | R | S | |
| 8 | Escherichia coli. | S | R | R | S | S | S | |
| 9 | Klebsiella spp. | S | R | S | S | R | R | |
| 10 |
Chicken chopping board/utensils
| Klebsiella spp. | S | R | S | S | S | R |
| 11 | Escherichia coli | R | R | S | R | S | R | |
| 12 | Escherichia coli. | S | R | S | S | R | R | |
| 13 | Escherichia coli. | S | S | S | S | S | R | |
| 14 | Klebsiella spp. | S | R | R | S | R | S | |
| 15 | Pseudomonas spp. | R | R | R | R | R | R | |
| 16 | Escherichia coli. | R | R | S | R | S | R | |
| 17 | Escherichia coli. | R | R | R | R | S | R | |
| 18 | Escherichia coli. | S | S | S | S | R | R | |
| 19 | Escherichia coli. | S | R | R | S | R | R | |
| 20 | Enterobacter spp. | R | R | R | R | R | R | |
| 21 | Escherichia coli. | R | R | R | R | R | R | |
| 22 |
Chicken dressing drum
| Staphylococcus aureus. | S | S | S | S | S | S |
| 23 | Escherichia coli. | S | R | S | S | S | R | |
| 24 | Pseudomonas spp. | S | R | R | S | S | R | |
| 25 | Pseudomonas spp. | S | R | R | S | S | R | |
| 26 | Citrobacter spp. | R | R | R | R | R | R | |
| 27 | Escherichia coli. | S | R | S | S | R | R |
Table 2 presents the antibiotic resistance and sensitivity profiles of six bacterial species—Escherichia coli, Pseudomonas spp., Citrobacter spp., Klebsiella spp., Enterobacter spp., and Staphylococcus aureus isolated from 27 samples collected from three different sources: beef chopping board/utensils, chicken chopping board/utensils, and chicken dressing drums. Among these, Escherichia coli was the most frequently isolated organism (11 isolates), showing notable resistance, particularly to azithromycin (AZM), ciprofloxacin (CIP), and tetracycline (TE), while maintaining relatively better sensitivity to cefixime (CFM) and cefuroxime (CXM). Pseudomonas spp. (6 isolates) exhibited high resistance, especially from chicken chopping boards, with some isolates being resistant to all six antibiotics tested. Citrobacter spp. (2 isolates) showed a high resistance rate, including complete resistance from chicken dressing drum samples. Klebsiella spp. (4 isolates) demonstrated moderate resistance, mainly to AZM and TE. Enterobacter spp. (2 isolates) showed stark contrast between sources, with full resistance in one chicken board isolate. Staphylococcus aureus (2 isolates) presented source-dependent variation, with one isolate from the beef board showing multidrug resistance and the other from the dressing drum being fully sensitive.
Table 3. Comparative Resistance of Antibiotics by Sample Sources.
| Bacterium | Beef chopping board/utensils | Chicken chopping board/utensils | Chicken dressing drum |
| Escherichia coli | 16.67% | 54.17% | 33.33% |
| Pseudomonas spp. | 50.00% | 100.00% | 50.00% |
| Citrobacter spp. | 50.00% | – | 100.00% |
| Klebsiella spp. | 50.00% | 41.67% | – |
| Enterobacter spp. | 16.67% | 100.00% | – |
| Staphylococcus aureus | 83.33% | – | 0.00% |
Table 3 illustrates the comparative antibiotic resistance percentages of six bacterial species isolated from three different sample sources: beef chopping board/utensils, chicken chopping board/utensils, and chicken dressing drum. Escherichia coli exhibited the highest resistance (54.17%) in chicken chopping board samples, while showing lower resistance in beef board (16.67%) and moderate resistance in dressing drum samples (33.33%). Pseudomonas spp. showed consistent high resistance, peaking at 100% in chicken boards and 50% in both beef board and drum samples. Citrobacter spp. demonstrated substantial resistance in beef boards (50%) and complete resistance (100%) in the dressing drum, though it was not found in chicken boards. Klebsiella spp. showed similar resistance levels in beef (50%) and chicken boards (41.67%) but was not detected in the drum. Enterobacter spp. displayed a sharp contrast, with low resistance in beef boards (16.67%) and full resistance (100%) in chicken boards. Staphylococcus aureus showed the highest resistance (83.33%) in beef board isolates, whereas it was fully sensitive (0% resistance) in chicken dressing drum samples and absent in chicken boards. Overall, the chicken chopping board/utensils harbored the most resistant isolates, highlighting a potential hotspot for antimicrobial-resistant bacteria in food preparation environments.
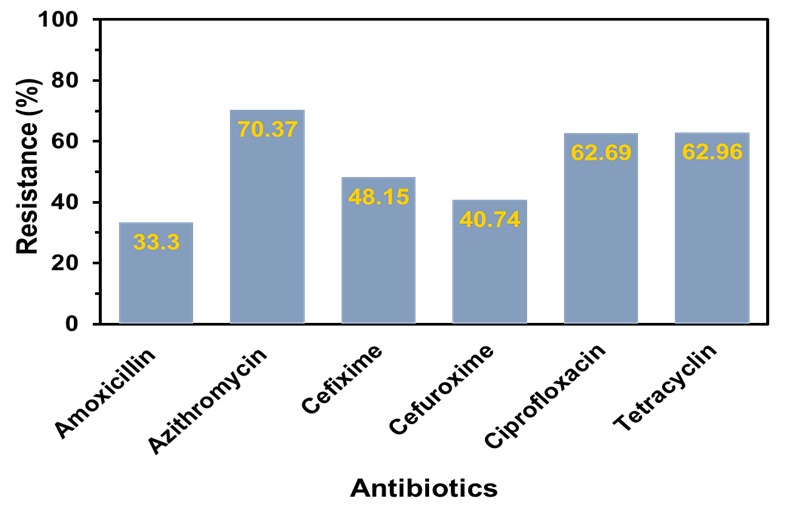
Figure 4. Comparative percentage of antibiotic resistance among bacteria isolated from beef wooden cutting boards, chicken cutting boards, and chicken drumsticks against various commercial antibiotics.
A comparative percentage of antibiotic resistance was demonstrated in Figure 4. From this data, it can be seen that the resistance of Azithromycin was quite significant (70.37%) followed by Tetracycline and Ciprofloxacin both are about 63% indicating these antibiotics may be ineffective in treating the infections caused by these bacteria. Moderate resistance was found in the case of Cefixime (48.15%), and the other two antibiotics showed the lowest resistance against the bacteria. The high levels of resistance observed, particularly to commonly used antibiotics such as azithromycin, ciprofloxacin, and tetracycline, may be partly attributed to inadequate food safety knowledge and poor hygiene practices among meat sellers. To further investigate this phenomenon, a cross-sectional survey was conducted to assess the sellers' demographic characteristics, food safety awareness, and hygiene-related practices.
3.3. Demographic Information, Hygiene Practices and Knowledge Level of Local Meat Vendors
A structured questionnaire-based survey was conducted to assess the safety and hygiene practices implemented by local meat vendors to minimize microbial contamination within shop premises and during meat handling. In total, 224 meat sellers participated in the study.
Table 4. Demographic and hygiene practices of respondents (n = 224).
| Characteristic | Category | Frequency (n) | Percentage (%) |
| Age | 20–30 years | 41 | 18.3% |
| 31–40 years | 46 | 20.5% | |
| 41–50 years | 79 | 35.3% | |
| Over 50 years | 58 | 25.9% | |
| Education | Primary | 111 | 49.6% |
| Secondary | 97 | 43.3% | |
| Graduate (or higher) | 16 | 7.1% | |
| Years in Business | 1–5 years | 23 | 10.3% |
| 6–10 years | 36 | 16.1% | |
| 11–15 years | 102 | 45.5% | |
| 16–20 years | 34 | 15.2% | |
| Over 20 years | 29 | 12.9% | |
Cleaning Practices (Chopping Board/Utensils) | Only Water | 81 | 36.2% |
| Only Cloths | 15 | 6.7% | |
| Water and Cloths | 95 | 42.4% | |
| Water and Sanitizer | 33 | 14.7% | |
| Frequency of Cleaning (Chopping Board/Utensils) | Never | 0 | 0% |
Rarely (1 time/week) | 5 | 2.2% | |
Sometimes (1–2 times / week) | 22 | 9.8% | |
Often (3–4 times / week) | 10 | 4.5% | |
Very Often (5–6 times / week) | 26 | 11.6% | |
| Always | 161 | 71.9% | |
Training on Food Safety And Hygiene | Yes | 26 | 11.6% |
| No | 198 | 88.4% | |
| Use of Protective Measures (e.g., Gloves, Aprons) | Never | 115 | 51.3% |
Rarely (1 time/week) | 16 | 7.1% | |
Sometimes (1–2 times / week) | 5 | 2.2% | |
Often (3–4 times / week) | 10 | 4.5% | |
Very Often (5–6 times / week) | 6 | 2.7% | |
| Always | 0 | 0% |
As shown in Table 4, sellers age range in between 41 to 50 years old was (35.3%), whereas nearly half of them (49.6%) had primary education. Most participants (45.5%) have been in business for over 10 years (11-15 years). While 42.8% used a combination of water and cloth to clean chopping boards and utensils, a significant portion (36.2%) still used only water. Cleaning frequency was high, with about 72.0% reporting they always clean their utensils. However, only 11.6% had received any food safety or hygiene training before or during doing business. The use of protective measures like gloves and aprons was very low, with 51.3% never using them and 0% always using them. This highlights a gap in hygiene training and consistent protective practices in the workplace.
Table 5. Knowledge level of local, meat retailers by demographic characteristics (n = 224).
| Characteristic | Category | Frequency (n) | Good Knowledge n (%) | Average Knowledge n (%) | Poor Knowledge n (%) | P-value |
| Age | 20–30 years | 41 | 19 (46.3%) | 13 (31.7%) | 9 (22.0%) | 0.021* |
| 31–40 years | 46 | 14 (30.4%) | 25 (54.3%) | 7 (15.2%) | ||
| 41–50 years | 79 | 31 (39.2%) | 28 (35.4%) | 20 (25.3%) | ||
| >50 years | 58 | 15 (25.9%) | 19 (32.8%) | 24 (41.4%) | ||
| Education | Primary | 111 | 13 (11.7%) | 57 (51.4%) | 41 (36.9%) | <0.001* |
| Secondary | 97 | 21 (21.6%) | 47 (48.5%) | 29 (29.9%) | ||
| Graduate | 16 | 11 (68.8%) | 5 (31.2%) | 0 (0.0%) | ||
| Years in Business | 1–5 years | 23 | 5 (21.7%) | 5 (21.7%) | 13 (56.5%) | 0.003* |
| 6–10 years | 36 | 8 (22.2%) | 17 (47.2%) | 11 (30.6%) | ||
| 11–15 years | 102 | 29 (28.4%) | 55 (53.9%) | 18 (17.6%) | ||
| 16–20 years | 34 | 18 (52.9%) | 11 (32.4%) | 5 (14.7%) | ||
| >20 years | 29 | 17 (58.6%) | 12 (41.4%) | 0 (0.0%) |
Table 5 presents the distribution of knowledge levels (Good, Average, Poor) among retailers based on age, education, and years in business. A significant association was observed between knowledge level and all three variables: age (p = 0.021), education (p < 0.001), and years in business (p = 0.003). Younger retailers (20–30 years) had a higher proportion with good knowledge (46.3%), while the oldest group (over 50 years) had the highest proportion with poor knowledge (41.4%). Retailers with higher education levels demonstrated better knowledge. Among graduates, 68.8% had good knowledge, and none were in the poor knowledge category, while only 11.7% of those with primary education had good knowledge. Moreover, retailers with more experience (over 20 years) had significantly better knowledge (58.6% good knowledge and 0% poor). Conversely, those with less than 5 years of experience had the highest proportion with poor knowledge (56.5%).
The findings of this study underscore significant microbial contamination and alarming levels of antibiotic resistance in local meat retail environments in Dhaka and nearby areas, Bangladesh. The high prevalence of Escherichia coli (37.04%), Pseudomonas spp. (22.22%), and Klebsiella spp. (14.81%) on meat-cutting surfaces, particularly chicken cutting boards, indicates critical hygiene deficiencies in food preparation practices. Chicken cutting boards exhibited the highest contamination rates (80% of samples showing bacterial growth), likely due to their frequent use and inadequate sanitization, as supported by previous studies highlighting poultry as a reservoir for foodborne pathogens [18]. The presence of Staphylococcus aureus and Citrobacter spp. further emphasizes the diversity of pathogens in these environments, posing risks of foodborne illnesses such as gastroenteritis and toxic-infections [19].
Antibiotic susceptibility testing revealed widespread resistance, with 70.37% of isolates resistant to azithromycin, 63% to tetracycline, and 63% to ciprofloxacin. These findings align with global reports of increasing AMR in foodborne pathogens, driven by the overuse of antibiotics in agriculture and clinical settings [20]. The high resistance to azithromycin and ciprofloxacin, commonly used in Bangladesh for both human and veterinary purposes, is particularly concerning, as it limits treatment options for infections caused by these bacteria [21]. The variability in resistance profiles across sample sources from chicken cutting boards showing the highest resistance rates (e.g., 100% for Pseudomonas spp.) suggests that specific handling practices and environmental factors in poultry processing may exacerbate AMR dissemination [22].
The cross-sectional survey of 224 meat sellers revealed critical gaps in food safety knowledge and hygiene practices. Only 11.6% of sellers had received formal training, and 51.3% never used protective gear such as gloves or aprons, consistent with findings from similar studies in developing countries [21]. The reliance on water alone (36.2%) or water with cloths (42.4%) for cleaning, without consistent use of sanitizers, likely contributes to persistent microbial contamination. Knowledge levels were significantly associated with education and experience, with graduates and sellers with over 20 years in business demonstrating better awareness, suggesting that targeted training could improve practices [23]. Without sustained adherence to proper food safety measures, achieving nutritional goals for school-going children and the younger generation as well as facing seasonal diseases like dengue fever, will remain challenging for an overpopulated country like Bangladesh [24, 25].
These findings underscore the need for comprehensive interventions. Enforcing hygiene regulations, implementing routine microbial monitoring, and mandating food safety training for meat handlers are crucial to minimizing contamination risks. However, limitations such as the study’s geographic focus on Dhaka and the absence of molecular analysis of resistance genes highlight the need for further research to better understand AMR mechanisms in retail meat settings.
This study revealed a disturbing level of microbial contamination and antimicrobial resistance in meat retail environments in Dhaka. Escherichia coli was the most frequently isolated organism, with many isolates showing resistance to commonly used antibiotics such as azithromycin, ciprofloxacin, and tetracycline. Chicken cutting boards were identified as the most contamination-prone surfaces, signaling a critical need for hygienic handling improvements. The cross-sectional survey further highlighted deficiencies in sellers’ food safety knowledge and hygienic practices, including the absence of formal training and minimal use of protective equipment. These findings suggest that the meat retail sector is a potential hotspot for the emergence and transmission of multidrug-resistant bacteria. To mitigate these risks, urgent and coordinated efforts are needed: policy-level enforcement of hygiene regulations, structured food safety training programs for meat handlers, and routine monitoring of antibiotic resistance trends in foodborne pathogens.
We extend our heartfelt gratitude to all those who contributed to the successful completion of this research project. We are immensely grateful to the participants, who allowed us to interview them, for the collection of data and samples.
This research was conducted with self-funding. Therefore, any kind of financial support was not received for this study.
The authors declare no conflict of interest. All aspects of this research were conducted impartially and independently. No financial or personal relationships with other people or organizations have influenced this work.
This study did not involve any experiments on human participants or animals; therefore, formal written informed consent was not required. However, oral consent was obtained from all participants prior to data collection, ensuring the privacy and confidentiality of participants were upheld throughout the study.